- Record: found
- Abstract: found
- Article: found
NFAT5 expression in bone marrow-derived cells enhances atherosclerosis and drives macrophage migration
Read this article at
Abstract
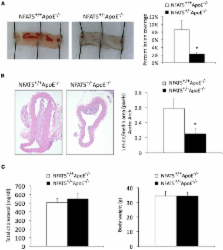
Objective: We have previously shown that the transcription factor, nuclear factor of activated T-cells 5 (NFAT5), regulates vascular smooth muscle cell phenotypic modulation, but the role of NFAT5 in atherosclerosis is unknown. Our main objective was to determine if NFAT5 expression in bone marrow (BM)-derived cells altered atherosclerotic development and macrophage function. Methods and Results: NFAT5 +/−ApoE −/− mice were generated for in vivo atherosclerosis studies. Following high fat diet feeding, en face analysis of the thoracic aorta established that genome-wide NFAT5 haploinsufficiency reduced atherosclerotic lesion formation by 73%. BM transplant studies revealed that transplantation of NFAT5 +/−ApoE −/− marrow into NFAT5 +/+ApoE −/− mice resulted in a similar 86% reduction in lesion formation. In vitro functional analysis of BM-derived macrophages demonstrated that NFAT5 is required for macrophage migration, which is a key event in the propagation of atherosclerosis. Conclusion: We have identified NFAT5 in BM-derived cells as a positive regulator of atherosclerotic lesion formation and macrophage function in the vasculature.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
The role of NFAT transcription factors in integrin-mediated carcinoma invasion.
- Record: found
- Abstract: found
- Article: not found
NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment.
- Record: found
- Abstract: found
- Article: not found