- Record: found
- Abstract: found
- Article: found
The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells
Read this article at
Abstract
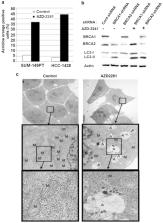
PARP inhibitors are considered promising anti-cancer agents and currently being tested in clinical trials in hereditary breast cancer patients harboring mutations in BRCA1 and BRCA2 genes. In this study, we investigated the antiproliferative effects and mechanism of PARP inhibitors ABT-888 (Veliparib), BSI-201 (Iniparib) and AZD228 (Olaparib) in breast cancer cell lines with BRCA1 or BRCA2 mutations and 9 different BRCA wild-type cell lines with BRCA1 allelic loss. We found that AZD2281 was the most potent in the PARP inhibitors and induces significant growth inhibition (~95%) in BRCA1 mutant (HCC-1937, MDA-MB-436, and SUM-149PT) and BRCA2 mutant (HCC-1428) cell lines. AZD2281 treatment also resulted in growth inhibition ranging from 20 to 50% in cells with BRCA1 allelic loss, including ER(+), HER2/Neu(+) and triple-negative breast cancer (TNBC) cells, but showed no effect in cells without with type BRCA without allelic loss. Knocking down of BRCA1 or BRCA2 in TNBC cells with BRCA1 allelic loss by RNA interference significantly enhanced AZD2281-induced growth inhibition and induced significant autophagy that was associated with mitophagy in cells with BRCA mutations. Inhibition of autophagy by gene knockdown significantly diminished AZD2281-induced mitophagy and apoptosis, indicating that autophagic process mediates some of the downstream effects of PARP inhibitors. In conclusion, our data provide the first evidence of PARP inhibitor AZD2281 autophagy and mitophagy in breast cancer cell lines with BRCA mutations or BRCA-allelic loss. In addition, our results indicate that the patients with BRCA1 allelic loss may also benefit from PARP inhibitor therapy if BRCA is further inhibited.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer.
- Record: found
- Abstract: found
- Article: not found