- Record: found
- Abstract: found
- Article: found
Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances
Read this article at
Abstract
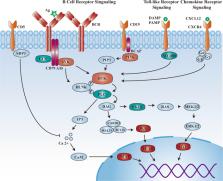
Bruton’s tyrosine kinase (BTK) inhibitor is a promising novel agent that has potential efficiency in B-cell malignancies. It took approximately 20 years from target discovery to new drug approval. The first-in-class drug ibrutinib creates possibilities for an era of chemotherapy-free management of B-cell malignancies, and it is so popular that gross sales have rapidly grown to more than 230 billion dollars in just 6 years, with annual sales exceeding 80 billion dollars; it also became one of the five top-selling medicines in the world. Numerous clinical trials of BTK inhibitors in cancers were initiated in the last decade, and ~73 trials were intensively announced or updated with extended follow-up data in the most recent 3 years. In this review, we summarized the significant milestones in the preclinical discovery and clinical development of BTK inhibitors to better understand the clinical and commercial potential as well as the directions being taken. Furthermore, it also contributes impactful lessons regarding the discovery and development of other novel therapies.
Related collections
Most cited references121
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib.
- Record: found
- Abstract: not found
- Article: not found