- Record: found
- Abstract: found
- Article: found
Hypoxia Inducible Factors’ Signaling in Pediatric High-Grade Gliomas: Role, Modelization and Innovative Targeted Approaches
Read this article at
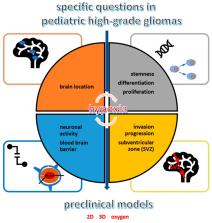
Abstract
The brain tumor microenvironment has recently become a major challenge in all pediatric cancers, but especially in brain tumors like high-grade gliomas. Hypoxia is one of the extrinsic tumor features that interacts with tumor cells, but also with the blood–brain barrier and all normal brain cells. It is the result of a dramatic proliferation and expansion of tumor cells that deprive the tissues of oxygen inflow. However, cancer cells, especially tumor stem cells, can endure extreme hypoxic conditions by rescheduling various genes’ expression involved in cell proliferation, metabolism and angiogenesis and thus, promote tumor expansion, therapeutic resistance and metabolic adaptation. This cellular adaptation implies Hypoxia-Inducible Factors (HIF), namely HIF-1α and HIF-2α. In pediatric high-grade gliomas (pHGGs), several questions remained open on hypoxia-specific role in normal brain during gliomagenesis and pHGG progression, as well how to model it in preclinical studies and how it might be counteracted with targeted therapies. Therefore, this review aims to gather various data about this key extrinsic tumor factor in pHGGs.
Related collections
Most cited references49

- Record: found
- Abstract: found
- Article: found
Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma
- Record: found
- Abstract: found
- Article: not found
Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer.
- Record: found
- Abstract: found
- Article: not found