- Record: found
- Abstract: found
- Article: found
Gene Correction of iPSCs from a Wiskott-Aldrich Syndrome Patient Normalizes the Lymphoid Developmental and Functional Defects
Read this article at
Summary
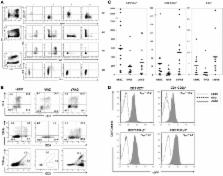
Wiskott-Aldrich syndrome (WAS) is an X-linked primary immunodeficiency disease caused by mutations in the gene encoding the WAS protein (WASp). Here, induced pluripotent stem cells (iPSCs) were derived from a WAS patient (WAS-iPSC) and the endogenous chromosomal WAS locus was targeted with a wtWAS-2A-eGFP transgene using zinc finger nucleases (ZFNs) to generate corrected WAS-iPSC (cWAS-iPSC). WASp and GFP were first expressed in the earliest CD34 +CD43 +CD45 − hematopoietic precursor cells and later in all hematopoietic lineages examined. Whereas differentiation to non-lymphoid lineages was readily obtained from WAS-iPSCs, in vitro T lymphopoiesis from WAS-iPSC was deficient with few CD4 +CD8 + double-positive and mature CD3 + T cells obtained. T cell differentiation was restored for cWAS-iPSCs. Similarly, defects in natural killer cell differentiation and function were restored on targeted correction of the WAS locus. These results demonstrate that the defects exhibited by WAS-iPSC-derived lymphoid cells were fully corrected and suggests the potential therapeutic use of gene-corrected WAS-iPSCs.
Highlights
-
•
Targeted endogenous knockin of Wiskott-Aldrich syndrome (WAS) transgene in WAS-iPSCs
-
•
Mutant WAS-iPSCs exhibited defective NK- and T-lymphoid cell development and function
-
•
Correction of WAS-iPSCs restored lymphoid cell development and function
-
•
These results suggest the potential therapeutic use of gene-corrected WAS-iPSCs
Abstract
In this article, Davis and colleagues investigated targeted gene correction of induced pluripotent stem cells derived from a patient with Wiskott-Aldrich syndrome (WAS). They employed a knockin strategy in which a WAS transgene construct was selectively integrated into the endogenous WAS locus. Lymphoid cell-specific development and function were selectively defective for WAS-iPSCs and restored for corrected iPSCs.
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: not found
Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells.
- Record: found
- Abstract: found
- Article: not found
Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells.
- Record: found
- Abstract: found
- Article: not found