- Record: found
- Abstract: found
- Article: found
Biphasic Alteration of Butyrylcholinesterase (BChE) During Prostate Cancer Development
Read this article at
Abstract
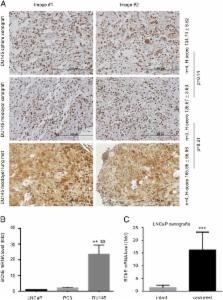
Butyrylcholinesterase (BChE) is a plasma enzyme that hydrolyzes ghrelin and bioactive esters, suggesting a role in modulating metabolism. Serum BChE is reduced in cancer patients. In prostate cancer (PC), the down-regulation is associated with disease recurrence. Nonetheless, how BChE is expressed in PC and its impact on PC remain unclear. We report here the biphasic changes of BChE expression in PC. In vitro, BChE expression was decreased in more tumorigenic PC stem-like cells (PCSLCs), DU145, and PC3 cells compared to less tumorigenic non-stem PCs and LNCaP cells. On the other hand, BChE was expressed at a higher level in LNCaP cells than immortalized but non-tumorigenic prostate epithelial BPH-1 cells. In vivo, BChE expression was up-regulated in DU145 xenografts compared to LNCaP xenografts; DU145 cell-derived lung metastases displayed comparable levels of BChE as subcutaneous tumors. Furthermore, LNCaP xenografts produced in castrated mice exhibited a significant increase of BChE expression compared to xenografts generated in intact mice. In patients, BChE expression was down-regulated in PCs (n = 340) compared to prostate tissues (n = 86). In two independent PC populations MSKCC (n = 130) and TCGA Provisional (n = 490), BChE mRNA levels were reduced from World Health Organization grade group 1 (WHOGG 1) PCs to WHOGG 3 PCs, followed by a significant increase in WHOGG 5 PCs. The up-regulation was associated with a reduction in disease-free survival ( P = .008). Collectively, we demonstrated for the first time a biphasic alteration of BChE, its down-regulation at early stage of PC and its up-regulation at advanced PC.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
PKM2 contributes to cancer metabolism.
- Record: found
- Abstract: found
- Article: not found
Butyrylcholinesterase as a prognostic marker: a review of the literature
- Record: found
- Abstract: found
- Article: not found