- Record: found
- Abstract: found
- Article: found
Characteristics of patients with chronic kidney disease and Type 2 diabetes initiating finerenone in the USA: a multi-database, cross-sectional study
Read this article at
Abstract
Aim:
Finerenone is safe and efficacious for treating patients with chronic kidney disease (CKD) and Type 2 diabetes (T2D). Evidence on the use of finerenone in clinical practice is lacking.
Objective:
To describe demographic and clinical characteristics of early adopters of finerenone in the United States, according to sodium-glucose cotransporter 2 inhibitor (SGLT2i) use and urine albumin–creatinine ratio (UACR) levels.
Methods:
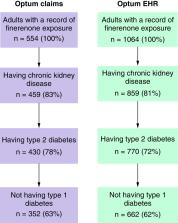
Multi-database, observational, cross-sectional study, using data from two US databases (Optum Claims and Optum EHR). Three cohorts were included: finerenone initiators with prior CKD-T2D, finerenone initiators with prior CKD-T2D and concomitant SGLT2i use, finerenone initiators with prior CKD-T2D stratified according to UACR.
Results:
In total, 1015 patients were included, 353 from Optum Claims and 662 from Optum EHR. Mean age was 72.0 and 68.4 years in Optum claims and EHR, respectively. Median eGFR was 44 and 44 ml/min/1.73 m 2; and median UACR was 132 (28–698)/365 (74–1185.4) mg/g, in Optum Claims and EHR, respectively. 70.5/70.4% were taking renin-angiotensin system inhibitors, 42.5/53.3% SGLT2i. Overall, 9.0/6.3% of patients had baseline UACR <30 mg/g, 15.0/20.2% had UACR 30–300 mg/g, and 14.4/27.6% had UACR >300 mg/g.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy
- Record: found
- Abstract: found
- Article: not found