- Record: found
- Abstract: found
- Article: found
It Takes Two: Dimerization Is Essential for the Broad-Spectrum Predatory and Defensive Activities of the Venom Peptide Mp1a from the Jack Jumper Ant Myrmecia pilosula
Read this article at
Abstract
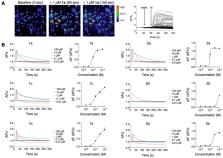
Ant venoms have recently attracted increased attention due to their chemical complexity, novel molecular frameworks, and diverse biological activities. The heterodimeric peptide ∆-myrtoxin-Mp1a (Mp1a) from the venom of the Australian jack jumper ant, Myrmecia pilosula, exhibits antimicrobial, membrane-disrupting, and pain-inducing activities. In the present study, we examined the activity of Mp1a and a panel of synthetic analogues against the gastrointestinal parasitic nematode Haemonchus contortus, the fruit fly Drosophila melanogaster, and for their ability to stimulate pain-sensing neurons. Mp1a was found to be both insecticidal and anthelmintic, and it robustly activated mammalian sensory neurons at concentrations similar to those reported to elicit antimicrobial and cytotoxic activity. The native antiparallel Mp1a heterodimer was more potent than heterodimers with alternative disulfide connectivity, as well as monomeric analogues. We conclude that the membrane-disrupting effects of Mp1a confer broad-spectrum biological activities that facilitate both predation and defense for the ant. Our structure–activity data also provide a foundation for the rational engineering of analogues with selectivity for particular cell types.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Scorpion venom components that affect ion-channels function.

- Record: found
- Abstract: found
- Article: found