- Record: found
- Abstract: found
- Article: not found
Remdesivir attenuates high fat diet (HFD)-induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING
Abstract
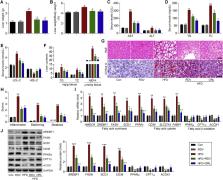
High-fat diet (HFD) is a predisposing factor for metabolic syndrome-related systemic inflammation and non-alcoholic fatty liver disease (NAFLD). However, there is still no effective therapeutic treatment for NAFLD. Here, we showed that remdesivir (RDV, GS-5734), as a broad-spectrum antiviral nucleotide prodrug with anti-inflammatory effects, was effective for attenuating HFD-induced metabolic disorder and insulin resistance. Results revealed that the liver weight, hepatic dysfunction and lipid accumulation were markedly increased compared with that of the Control group, while that of the RDV group exhibited significant reduction, accompanied by the improved signaling pathway regulating fatty acid metabolism. In agreement with reduced lipid deposition, RDV supplementation suppressed the systematic and hepatic inflammation, as evidenced by reduction of inflammatory cytokines and the blockage of nuclear factor κB (NF-κB) signaling. In addition, stimulator of interferon genes (STING) and its down-streaming factor interferon regulatory factor 3 (IRF3) were greatly increased in livers of HFD-fed mice, which were considerably restrained by RDV treatment. The in vitro analysis suggested that RDV functioned as an inhibitor of STING, contributing to the suppression of dyslipidemia and inflammation induced by palmitate (PA). However, PA-triggered lipid deposition and inflammatory response was further accelerated in hepatocytes with STING over-expression. Notably, RDV-attenuated lipid disorder and inflammation were significantly abrogated by the over-expression of STING in PA-stimulated hepatocytes. Taken together, these findings indicated that RDV exhibited protective effects against NAFLD development mainly through repressing STING signaling, and thus could be considered as a potential therapeutic strategy.
Highlights
-
•
Remdesivir attenuates metabolic syndrome in HFD-fed mice.
-
•
Remdesivir alleviates hepatic function and lipid accumulation in HFD-induced mice.
-
•
Remdesivir inhibits inflammatory response in liver of HFD-fed mice.
-
•
Remdesivir-regulated lipid accumulation and inflammation is dependent on STING in PA-incubated hepatocytes.
Related collections
Most cited references18

- Record: found
- Abstract: found
- Article: found
Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir
- Record: found
- Abstract: found
- Article: not found
The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3.
- Record: found
- Abstract: found
- Article: not found
