- Record: found
- Abstract: found
- Article: found
GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process
Read this article at
Abstract
Simple Summary
The knowledge of physiological events associated with canine reproduction involving oocyte developmental potential is essential to increase the success of reproductive biotechnologies in this species. In mammals, the oocytes are closely surrounded by a group of cells known as the cumulus cells. Although it is not well-known how these cells interact with the oocyte to promote maturation, they may provide important answers concerning oocyte development. The competence to undergo expansion is a unique characteristic of cumulus cells which is critical for normal oocyte maturation, however, the complete expansion of these cells takes longer in canines, which has been associated with the lengthy maturation process of the oocyte. Growth Differentiation Factor 9 (GDF-9) and Bone Morphogenetic Protein 15 (BMP-15) are described as relevant players in the oocyte–cumulus cells’ regulatory mechanisms. Cumulus cells express many important genes from a very early stage, therefore, we proposed to study the gene expression of GDF-9 and BMP-15 in canine cumulus cells in relation to cumulus expansion and the maturation process. We demonstrate, for the first time, that these genes are differentially expressed in canine cumulus cells throughout the estrous cycle and that this expression is related to cumulus expansion and maturity status, suggesting specific regulation.
Abstract
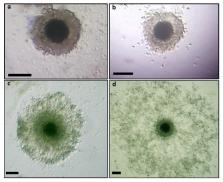
The competence to undergo expansion is a characteristic of cumulus cells (CCs). The aim was to investigate the expression of GDF-9 and BMP-15 mRNA in canine cumulus cells in relation to cumulus expansion and meiotic development over the estrous cycle. CCs were recovered from nonmatured and in vitro-matured (IVM) dog cumulus oocyte complexes (COCs), which were obtained from antral follicles at different phases of the estrous cycle. Quantitative real-time polymerase chain reaction (q-PCR) was used to evaluate the relative abundance of GDF-9 and BMP-15 transcripts from the CCs with or without signs of expansion. The results were evaluated by ANOVA and logistic regression. The maturity of the oocyte and the expansion process affected the mRNA levels in CCs. There were differences ( p < 0.05) in GDF-9 and BMP-15 gene expression in CCs isolated from nonmatured COCs when comparing the reproductive phases. Lower mRNA levels ( p < 0.05) were observed in anestrus and proestrus in comparison to those in estrus and diestrus. In contrast, when comparing GDF-9 mRNA levels in IVM COCs, no differences were found among the phases of the estrous cycle in expanded and nonexpanded CCs ( p < 0.05). However, the highest ( p < 0.05) BMP-15 gene expression in CCs that did not undergo expansion was exhibited in anestrus and the lowest ( p < 0.05) expression was observed in estrus in expanded CCs. Although the stage of the estrous cycle did not affect the second metaphase (MII )rates, the expanded CCs obtained at estrus coexisted with higher percentages of MII ( p < 0.05). In conclusion, the differential expression patterns of GDF-9 and BMP-15 mRNA transcripts might be related to cumulus expansion and maturation processes, suggesting specific regulation and temporal changes in their expression.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality.
- Record: found
- Abstract: found
- Article: not found
Oocyte control of ovarian follicular development and function in mammals.
- Record: found
- Abstract: found
- Article: not found