- Record: found
- Abstract: found
- Article: found
Pilot study to evaluate hypercoagulation and inflammation using rotational thromboelastometry and calprotectin in COVID-19 patients
Read this article at
Abstract
Introduction
Abnormal coagulation and inflammation are hallmarks of SARs-COV-19. Stratifying affected patients on admission to hospital may help identify those who at are risk of developing severe disease early on. Rotational Thromboelastometry (ROTEM) is a point of care test that can be used to measure abnormal coagulation and calprotectin is a measure of inflammation.
Aim
Assess if ROTEM can measure hypercoagulability on admission and identify those who will develop severe disease early on. Assess if calprotectin can measure inflammation and if there is a correlation with ROTEM and calprotectin.
Methods
COVID-19 patients were recruited on admission and ROTEM testing was undertaken daily for a period of 7 days. Additionally inflammatory marker calprotectin was also tested for the same period.
Results
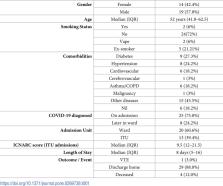
33 patients were recruited to the study out of which 13 were admitted to ITU and 20 were treated on the ward. ROTEM detected a hypercoagulable state on admission but did not stratify between those admitted to a ward or escalated to ITU. Calprotectin levels were raised but there was no statistical difference (p = 0.73) between patients admitted to a ward or escalated to ITU. Significant correlations were observed between FIBA5 (r = 0.62; p<0.00), FIBCFT (r = -0.57; p<0.00), FIBMCF (r = 0.64; p<0.00) and INMCF (r = 0.57; p<0.00) and calprotectin.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia
- Record: found
- Abstract: found
- Article: found