- Record: found
- Abstract: found
- Article: found
Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy
Read this article at
Abstract
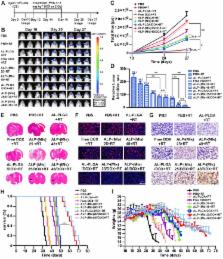
Treatment of malignant glioma is a challenge facing cancer therapy. In addition to surgery, and chemotherapy, radiotherapy (RT) is one of the most effective modalities of glioma treatment. However, there are two crucial challenges for RT facing malignant glioma therapy: first, gliomas are known to be resistant to radiation due to their intratumoral hypoxia; second, radiosensitizers may exhibit a lack of target specificity, which may cause a lower concentration of radiosensitizers in tumors and toxic side effects in normal tissues. Thus, novel angiopep-2-lipid-poly-(metronidazoles)n (ALP-(MIs)n) hypoxic radiosensitizer-polyprodrug nanoparticles (NPs) were designed to enhance the radiosensitizing effect on gliomas.
Methods: In this study, different degrees and biodegradabilites of hypoxic radiosensitizer MIs-based polyprodrug (P-(MIs)n) were synthesized as a hydrophobic core. P-(MIs)n were mixed with DSPE-PEG2000, angiopep-2-DSPE-PEG2000 and lecithin to self-assemble ALP-(MIs)n through a single-step nanoprecipitation method. The ALP-(MIs)n encapsulate doxorubicin (DOX) (ALP-(MIs)n/DOX) and provoke the release of DOX under hypoxic conditions for glioma chemo- and radiotherapy. In vivo glioma targeting was tested in an orthotopic glioma using live animal fluorescence/bioluminescence imaging. The effect on sensitization to RT of ALP-(MIs)n and the combination of chemotherapy and RT of ALP-(MIs)n/DOX for glioma treatment were also investigated both in vitro and in vivo.
Results: ALP-(MIs)n/DOX effectively accumulated in gliomas and could reach the hypoxic glioma site after systemic in vivo administration. These ALP-(MIs)n showed a significant radiosensitizing effect on gliomas and realized combination chemotherapy and RT for glioma treatment both in vitro and in vivo.
Conclusions: In summary, we constructed a lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for enhancing the RT sensitivity of gliomas and achieving the combination of radiation and chemotherapy for gliomas.
Related collections
Most cited references49

- Record: found
- Abstract: found
- Article: found
γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin
- Record: found
- Abstract: found
- Article: not found
Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery.
- Record: found
- Abstract: found
- Article: not found