- Record: found
- Abstract: found
- Article: found
Mice lacking perforin have improved regeneration of the injured femoral nerve
Read this article at
Abstract
Abstract
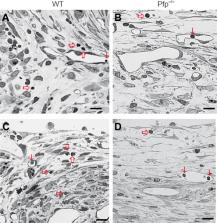
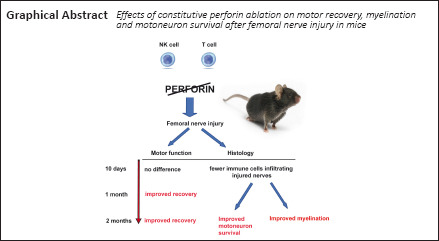
The role that the immune system plays after injury of the peripheral nervous system is still not completely understood. Perforin, a natural killer cell- and T-lymphocyte-derived enzyme that mediates cytotoxicity, plays important roles in autoimmune diseases, infections and central nervous system trauma, such as spinal cord injury. To dissect the roles of this single component of the immune response to injury, we tested regeneration after femoral nerve injury in perforin-deficient (Pfp –/–) and wild-type control mice. Single frame motion analysis showed better motor recovery in Pfp –/– mice compared with control mice at 4 and 8 weeks after injury. Retrograde tracing of the motoneuron axons regrown into the motor nerve branch demonstrated more correctly projecting motoneurons in the spinal cord of Pfp –/– mice compared with wild-types. Myelination of regrown axons measured by g-ratio was more extensive in Pfp –/– than in wild-type mice in the motor branch of the femoral nerve. Pfp –/– mice displayed more cholinergic synaptic terminals around cell bodies of spinal motoneurons after injury than the injured wild-types. We histologically analyzed lymphocyte infiltration 10 days after surgery and found that in Pfp –/– mice the number of lymphocytes in the regenerating nerves was lower than in wild-types, suggesting a closed blood-nerve barrier in Pfp –/– mice. We conclude that perforin restricts motor recovery after femoral nerve injury owing to decreased survival of motoneurons and reduced myelination.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Perforin and granzymes: function, dysfunction and human pathology.
- Record: found
- Abstract: found
- Article: not found
The cellular and molecular basis of peripheral nerve regeneration.
- Record: found
- Abstract: found
- Article: not found