- Record: found
- Abstract: found
- Article: found
Integration of Signaling Pathways with the Epigenetic Machinery in the Maintenance of Stem Cells
Read this article at
Abstract
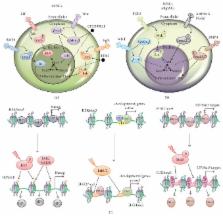
Stem cells balance their self-renewal and differentiation potential by integrating environmental signals with the transcriptional regulatory network. The maintenance of cell identity and/or cell lineage commitment relies on the interplay of multiple factors including signaling pathways, transcription factors, and the epigenetic machinery. These regulatory modules are strongly interconnected and they influence the pattern of gene expression of stem cells, thus guiding their cellular fate. Embryonic stem cells (ESCs) represent an invaluable tool to study this interplay, being able to indefinitely self-renew and to differentiate towards all three embryonic germ layers in response to developmental cues. In this review, we highlight those mechanisms of signaling to chromatin, which regulate chromatin modifying enzymes, histone modifications, and nucleosome occupancy. In addition, we report the molecular mechanisms through which signaling pathways affect both the epigenetic and the transcriptional state of ESCs, thereby influencing their cell identity. We propose that the dynamic nature of oscillating signaling and the different regulatory network topologies through which those signals are encoded determine specific gene expression programs, leading to the fluctuation of ESCs among multiple pluripotent states or to the establishment of the necessary conditions to exit pluripotency.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation.
- Record: found
- Abstract: found
- Article: not found
Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found