- Record: found
- Abstract: found
- Article: not found
Anti-metastatic Inhibitors of Lysyl Oxidase (LOX): Design and Structure–Activity Relationships
Read this article at
Abstract
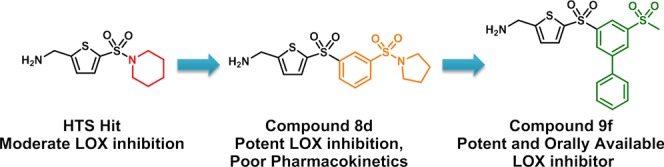
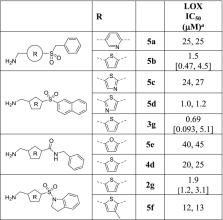
Lysyl oxidase (LOX) is a secreted copper-dependent amine oxidase that cross-links collagens and elastin in the extracellular matrix and is a critical mediator of tumor growth and metastatic spread. LOX is a target for cancer therapy, and thus the search for therapeutic agents against LOX has been widely sought. We report herein the medicinal chemistry discovery of a series of LOX inhibitors bearing an aminomethylenethiophene (AMT) scaffold. High-throughput screening provided the initial hits. Structure–activity relationship (SAR) studies led to the discovery of AMT inhibitors with sub-micromolar half-maximal inhibitory concentrations (IC 50) in a LOX enzyme activity assay. Further SAR optimization yielded the orally bioavailable LOX inhibitor CCT365623 with good anti-LOX potency, selectivity, pharmacokinetic properties, as well as anti-metastatic efficacy.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell.
- Record: found
- Abstract: found
- Article: not found
Lysyl oxidase: an oxidative enzyme and effector of cell function.
- Record: found
- Abstract: found
- Article: not found
