- Record: found
- Abstract: found
- Article: found
Crystal Structure of Calcium Binding Protein-5 from Entamoeba histolytica and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
Read this article at
Abstract
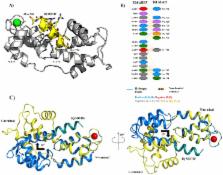
Entamoeba histolytica is the etiological agent of human amoebic colitis and liver abscess, and causes a high level of morbidity and mortality worldwide, particularly in developing countries. There are a number of studies that have shown a crucial role for Ca 2+ and its binding protein in amoebic biology. EhCaBP5 is one of the EF hand calcium-binding proteins of E. histolytica. We have determined the crystal structure of EhCaBP5 at 1.9 Å resolution in the Ca 2+-bound state, which shows an unconventional mode of Ca 2+ binding involving coordination to a closed yet canonical EF-hand motif. Structurally, EhCaBP5 is more similar to the essential light chain of myosin than to Calmodulin despite its somewhat greater sequence identity with Calmodulin. This structure-based analysis suggests that EhCaBP5 could be a light chain of myosin. Surface plasmon resonance studies confirmed this hypothesis, and in particular showed that EhCaBP5 interacts with the IQ motif of myosin 1B in calcium independent manner. It also appears from modelling of the EhCaBP5-IQ motif complex that EhCaBP5 undergoes a structural change in order to bind the IQ motif of myosin. This specific interaction was further confirmed by the observation that EhCaBP5 and myosin 1B are colocalized in E. histolytica during phagocytic cup formation. Immunoprecipitation of EhCaBP5 from total E. histolytica cellular extract also pulls out myosin 1B and this interaction was confirmed to be Ca 2+ independent. Confocal imaging of E. histolytica showed that EhCaBP5 and myosin 1B are part of phagosomes. Overexpression of EhCaBP5 increases slight rate (∼20%) of phagosome formation, while suppression reduces the rate drastically (∼55%). Taken together, these experiments indicate that EhCaBP5 is likely to be the light chain of myosin 1B. Interestingly, EhCaBP5 is not present in the phagosome after its formation suggesting EhCaBP5 may be playing a regulatory role.
Author Summary
Entamoeba histolytica is the etiologic agent of amoebiasis, a major cause of morbidity and mortality in developing countries. The genome of this organism encodes 27 EF-hand containing calcium binding proteins suggesting an intricate Ca 2+ signalling system that plays crucial role in phagocytosis and pathogenesis. Calcium binding protein-5 (EhCaBP5) is one of these CaBPs that displays sequence similarity with Calmodulin (CaM) but has only two possible calcium binding EF-hand loops in two separate domains. Interestingly crystal structure of EhCaPB5 showed more structural similarity with essential light chain (ELC) of myosin than that of CaM. The binding studies of EhCaBP5 with IQ motif peptides of myosins, showed that it interacts with IQ motif of unconventional Myosin IB. A number of experiments were carried out to show that EhCaBP5 indeed binds myosin IB and that this binding is Ca 2+ independent. We also show here that EhCaBP5 participates in erythrophagocytosis and that its role in phagocytosis is different from that of EhCaBP3, another myosin 1B interacting calcium binding protein of E. histolytica. Our results presented here and in a number of other reports point towards a unique phagocytic pathway involving a number of calcium binding proteins in E. histolytica.
Related collections
Most cited references31
- Record: found
- Abstract: not found
- Article: not found
Solvent content of protein crystals.
- Record: found
- Abstract: found
- Article: not found