- Record: found
- Abstract: found
- Article: not found
Novel bovine viral diarrhea virus (BVDV) virus-like particle vaccine candidates presenting the E2 protein using the SpyTag/SpyCatcher system induce a robust neutralizing antibody response in mice
Read this article at
Abstract
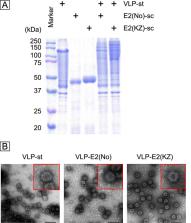
Bovine viral diarrhea virus (BVDV) is a pathogen of commercial consequence in cattle. Although many modified live and killed vaccines are commercially available, their drawbacks precipitate the need for new effective vaccines. Virus-like particles (VLPs) are a safe and powerful technology used in several human and veterinary vaccines; however, it is difficult to produce large amounts of BVDV VLPs. In this study, we generated red-spotted grouper nervous necrosis virus (RGNNV) VLPs presenting the BVDV E2 protein (domain I to IIIb) of the Nose (BVDV-1) or KZ-91-CP (BVDV-2) strain by exploiting SpyTag/SpyCatcher technology. Mice immunized twice with 30 μg of RGNNV VLPs conjugated with 10 μg of E2 proteins of the Nose or KZ-91-CP strain with a 14-day interval elicited high (1:512,000 to 1:1,024,000) and moderate (1:25,600 to 1:102,400) IgG titers against E2 proteins of homologous and heterologous strains, respectively. In addition, this prime-boost regimen induced strong (1:800 to 1:3,200) and weak (~1:10) neutralization titers against homologous and heterologous BVDV strains, respectively. Our results indicate that conjugation of the E2 protein to RGNNV VLPs strongly enhances the antigenicity of the E2 protein and that RGNNV VLPs presenting the E2 protein are promising BVDV vaccine candidates.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns.
- Record: found
- Abstract: found
- Article: not found
Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin.

- Record: found
- Abstract: found
- Article: found
