- Record: found
- Abstract: found
- Article: found
The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target
Read this article at
Abstract
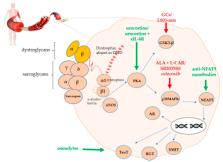
In Duchenne muscular dystrophy (DMD), the absence of dystrophin from the dystrophin-associated protein complex (DAPC) causes muscle membrane instability, which leads to myofiber necrosis, hampered regeneration, and chronic inflammation. The resulting disabled DAPC-associated cellular pathways have been described both at the molecular and the therapeutical level, with the Toll-like receptor nuclear factor kappa-light-chain-enhancer of activated B cells pathway (NF-ƘB), Janus kinase/signal transducer and activator of transcription proteins, and the transforming growth factor-β pathways receiving the most attention. In this review, we specifically focus on the protein kinase A/ mitogen-activated protein kinase/nuclear factor of activated T-cells 5/organic osmolytes (PKA-p38MAPK-NFAT5-organic osmolytes) pathway. This pathway plays an important role in osmotic homeostasis essential to normal cell physiology via its regulation of the influx/efflux of organic osmolytes. Besides, NFAT5 plays an essential role in cell survival under hyperosmolar conditions, in skeletal muscle regeneration, and in tissue inflammation, closely interacting with the master regulator of inflammation NF-ƘB. We describe the involvement of the PKA-p38MAPK-NFAT5-organic osmolytes pathway in DMD pathophysiology and provide a clear overview of which therapeutic molecules could be of potential benefit to DMD patients. We conclude that modulation of the PKA-p38MAPK-NFAT5-organic osmolytes pathway could be developed as supportive treatment for DMD in conjunction with genetic therapy.
Related collections
Most cited references154
- Record: found
- Abstract: found
- Article: not found
Dystrophin: the protein product of the Duchenne muscular dystrophy locus.

- Record: found
- Abstract: found
- Article: found
The Therapeutic Potential of Nanobodies
- Record: found
- Abstract: found
- Article: not found