- Record: found
- Abstract: found
- Article: found
Association of β-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction : A Secondary Analysis of the TOPCAT Trial
Read this article at
Key Points
Question
Is there an association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction?
Findings
In this secondary analysis of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist randomized clinical trial of spironolactone for patients with heart failure with a preserved ejection fraction of 50% or greater, β-blocker use was associated with a higher risk of heart failure hospitalizations compared with patients not taking β-blockers. This association was not present among patients with an ejection fraction between 45% and 49%.
Abstract
Importance
β-Blockers are prescribed to most patients with heart failure (HF) with a preserved ejection fraction (HFpEF), but their effect on HFpEF remains unclear.
Objective
To determine the association of β-blocker use with HF hospitalizations and cardiovascular disease (CVD) mortality, overall and in strata of patients with an ejection fraction (EF) of 50% or greater or less than 50%.
Design, Setting, and Participants
For 1761 participants from North and South America enrolled in the multicenter, double-blinded Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist randomized clinical trial of spironolactone for patients with HFpEF between August 10, 2006, and January 31, 2012, the association of baseline β-blocker use with HF hospitalization and CVD mortality was analyzed using unadjusted and adjusted Cox proportional hazards regression models, overall and in strata of patients with an EF of 50% or greater or less than 50%. Participants had symptomatic HF with a left ventricular EF of 45% or greater, with enrollment based on either hospitalization attributed to decompensated HF in the prior year or elevated natriuretic peptide levels. Statistical analysis was performed from January 31 to May 2, 2019.
Results
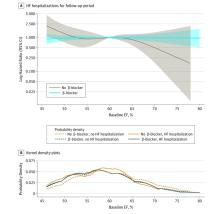
Among 1761 participants included in the analysis (879 women and 882 men; mean [SD] age, 71.5 [9.6] years), 1394 (79.2%) reported β-blocker use and 1567 (89.0%) had an EF of 50% or greater. Hospitalizations for HF occurred for 399 participants (22.7%), and CVD mortality occurred for 229 participants (13.0%). Use of β-blockers was associated with a higher risk of HF hospitalization among patients with HFpEF with an EF of 50% or greater (hazard ratio, 1.74 [95% CI, 1.28-2.37]; P < .001) but not among patients with an EF between 45% and 49% (hazard ratio, 0.68 [95% CI, 0.28-1.63]; P = .39). There was a significant interaction between β-blocker use and EF threshold for incident HF hospitalizations ( P = .03). Use of β-blockers was not associated with a change in CVD mortality.
Abstract
This secondary analysis of the TOPCAT randomized clinical trial examines the association of β-blocker use with heart failure (HF) hospitalizations and cardiovascular disease (CVD) mortality, overall and in strata of patients with an ejection fraction (EF) of 50% or greater or less than 50%.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Irbesartan in patients with heart failure and preserved ejection fraction.
- Record: found
- Abstract: found
- Article: not found
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial.
- Record: found
- Abstract: found
- Article: not found