- Record: found
- Abstract: found
- Article: found
Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm
Read this article at
Abstract
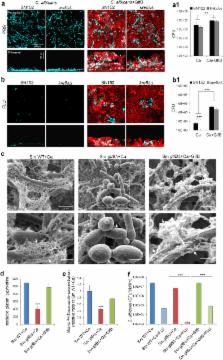
Fungal–bacterial interactions generate unique biofilms that cause many infections in humans. Candida albicans interact with Streptococcus mutans in dental biofilms associated with severe childhood tooth-decay, a prevalent pediatric oral disease. Current modalities are ineffective and primarily based on antimicrobial monotherapies despite the polymicrobial nature of the infection. Here, we show that the combination of clinically used topical antifungal fluconazole with povidone iodine (PI) can completely suppress C. albicans carriage and mixed-biofilm formation without increasing bacterial killing activity in vivo. We unexpectedly found that the inclusion of PI enhanced fluconazole efficacy by potently disrupting the assembly of a protective bacterial exopolysaccharide (EPS) matrix through inhibition of α-glucan synthesis by S. mutans exoenzyme (GtfB) bound on the fungal surface. Further analyses revealed that the EPS produced in situ directly bind and sequester fluconazole, reducing uptake and intracellular transportation of the drug. Conversely, inhibition of GtfB activity by PI, enzymatic degradation of the α-glucan matrix or co-culturing with gtfB-defective S. mutans re-established antifungal susceptibility. Hence, topical antifungal has limitations in mixed oral biofilms due to enhanced C. albicans tolerance to fluconazole afforded by the shielding effect of bacterial-derived EPS. The data provide new insights for treatment of C. albicans in cross-kingdom biofilms, indicating that EPS inhibitors may be required for enhanced killing efficacy and optimal anti-biofilm activity.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance.
- Record: found
- Abstract: found
- Article: not found