- Record: found
- Abstract: found
- Article: found
Growth Hormone Is Secreted by Normal Breast Epithelium upon Progesterone Stimulation and Increases Proliferation of Stem/Progenitor Cells

Read this article at
Summary
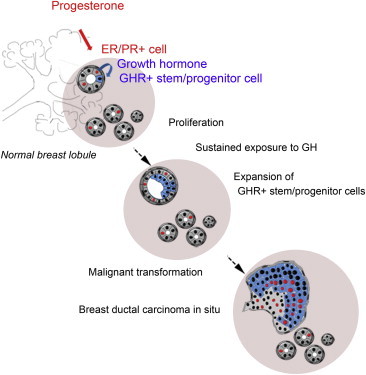
Using in vitro and in vivo experimental systems and in situ analysis, we show that growth hormone (GH) is secreted locally by normal human mammary epithelial cells upon progesterone stimulation. GH increases proliferation of a subset of cells that express growth hormone receptor (GHR) and have functional properties of stem and early progenitor cells. In 72% of ductal carcinoma in situ lesions, an expansion of the cell population that expresses GHR was observed, suggesting that GH signaling may contribute to breast cancer development.
Graphical Abstract
Highlights
Abstract
Growth hormone (GH) is secreted locally by normal human mammary epithelial cells upon progesterone stimulation. GH signaling increases proliferation of mammary stem and progenitor cells. Analysis of ductal carcinoma in situ lesions shows an expansion of the cell population that expresses GH receptor, suggesting that GH signaling may contribute to breast cancer development.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells.
- Record: found
- Abstract: found
- Article: not found
Reconstruction of functionally normal and malignant human breast tissues in mice.
- Record: found
- Abstract: found
- Article: not found