- Record: found
- Abstract: found
- Article: not found
Tunneling Probability Increases with Distance in Junctions Comprising Self-Assembled Monolayers of Oligothiophenes

Read this article at
Abstract
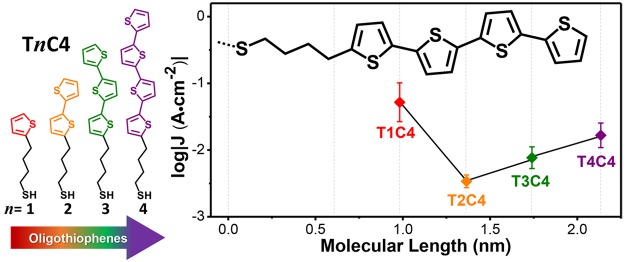
Molecular tunneling junctions should enable the tailoring of charge-transport at the quantum level through synthetic chemistry but are hindered by the dominance of the electrodes. We show that the frontier orbitals of molecules can be decoupled from the electrodes, preserving their relative energies in self-assembled monolayers even when a top-contact is applied. This decoupling leads to the remarkable observation of tunneling probabilities that increase with distance in a series of oligothiophenes, which we explain using a two-barrier tunneling model. This model is generalizable to any conjugated oligomers for which the frontier orbital gap can be determined and predicts that the molecular orbitals that dominate tunneling charge-transport can be positioned via molecular design rather than by domination of Fermi-level pinning arising from strong hybridization. The ability to preserve the electronic structure of molecules in tunneling junctions facilitates the application of well-established synthetic design rules to tailor the properties of molecular-electronic devices.
Related collections
Most cited references42
- Record: found
- Abstract: not found
- Article: not found
Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film
- Record: found
- Abstract: found
- Article: not found
Molecular-Scale Electronics: From Concept to Function.
- Record: found
- Abstract: found
- Article: found
