- Record: found
- Abstract: found
- Article: found
Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis
Read this article at
Abstract
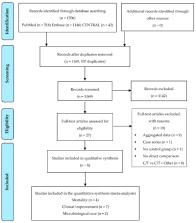
Ceftolozane-tazobactam (C/T) is a combination of an advanced-generation cephalosporin (ceftolozane) with a β-lactamase inhibitor (tazobactam). It is approved for the treatment of complicated urinary-tract/intra-abdominal infections and hospital-acquired/ventilator-associated pneumonia. This systematic review and meta-analysis (registered prospectively on PROSPERO, no. CRD42019134099, on 20 January 2020) aimed to evaluate the effectiveness of C/T combination therapy compared to C/T monotherapy for the treatment of severe infections and to describe the prevalence of microorganisms in the included studies. We retrieved literature from PubMed, EMBASE, and CENTRAL, until 26 November 2020. Eligible studies were both randomised trials and nonrandomised studies with a control group, published in the English language and peer-reviewed journals. The primary outcome was all-cause mortality; secondary outcomes were (i) clinical improvement and (ii) microbiological cure. Eight nonrandomised studies were included in the qualitative synthesis: Seven retrospective cohort studies and one case-control study. The meta-analysis of the four studies evaluating all-cause mortality (in total 148 patients: 87 patients treated with C/T alone and 61 patients treated with C/T combination therapy) showed a significant reduction of mortality in patients receiving C/T combination therapy, OR: 0.31, 95% CI: 0.10–0.97, p = 0.045. Conversely, the meta-analysis of the studies evaluating clinical improvement and microbiological cure showed no differences in C/T combination therapy compared to C/T monotherapy. The most consistent data come from the analysis of the clinical improvement, n = 391 patients, OR: 0.97, 95% CI: 0.54–1.74, p = 0.909. In 238 of the 391 patients included (60.8%), C/T was used for the treatment of infections caused by Pseudomonas aeruginosa.
Related collections
Most cited references16
- Record: found
- Abstract: not found
- Article: not found
Combating antimicrobial resistance: policy recommendations to save lives.
- Record: found
- Abstract: found
- Article: not found
Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance
- Record: found
- Abstract: found
- Article: not found