- Record: found
- Abstract: found
- Article: found
Multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke. The effect of periprocedural medication: acetylsalicylic acid, unfractionated heparin, both, or neither (MR CLEAN-MED). Rationale and study design
Read this article at
Abstract
Background
Despite evidence of a quite large beneficial effect of endovascular treatment (EVT) for ischemic stroke caused by anterior circulation large vessel occlusion, many patients do not recover even after complete recanalization. To some extent, this may be attributable to incomplete microvascular reperfusion, which can possibly be improved by antiplatelet agents and heparin. It is unknown whether periprocedural antithrombotic medication in patients treated with EVT improves functional outcome. The aim of this study is to assess the effect of acetylsalicylic acid (ASA) and unfractionated heparin (UFH), alone, or in combination, given to patients with an ischemic stroke caused by an intracranial large vessel occlusion in the anterior circulation during EVT.
Methods
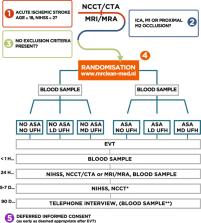
MR CLEAN-MED is a multicenter phase III trial with a prospective, 2 × 3 factorial randomized, open label, blinded end-point (PROBE) design, which aims to enroll 1500 patients. The trial is designed to evaluate the effect of intravenous ASA (300 mg), UFH (low or moderate dose), both or neither as adjunctive therapy to EVT. We enroll adult patients with a clinical diagnosis of stroke (NIHSS ≥ 2) and with a confirmed intracranial large vessel occlusion in the anterior circulation on CTA or MRA, when EVT within 6 h from symptom onset is indicated and possible. The primary outcome is the score on the modified Rankin Scale (mRS) at 90 days. Treatment effect on the mRS will be estimated with ordinal logistic regression analysis, with adjustment for main prognostic variables. Secondary outcomes include stroke severity measured with the NIHSS at 24 h and at 5–7 days, follow-up infarct volume, symptomatic intracranial hemorrhage (sICH), and mortality.
Discussion
Clinical equipoise exists whether antithrombotic medication should be administered during EVT for a large vessel occlusion, as ASA and/or UFH may improve functional outcome, but might also lead to an increased risk of sICH. When one or both of the study treatments show the anticipated effect on outcome, we will be able to improve outcome of patients treated with EVT by 5%. This amounts to more than 50 patients annually in the Netherlands, more than 1800 in Europe, and more than 1300 in the USA.
Related collections
Most cited references40
- Record: found
- Abstract: not found
- Article: not found
The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy.
- Record: found
- Abstract: found
- Article: not found
Neutrophil extracellular traps in ischemic stroke thrombi.
- Record: found
- Abstract: found
- Article: not found