- Record: found
- Abstract: found
- Article: found
Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation
Read this article at
Abstract

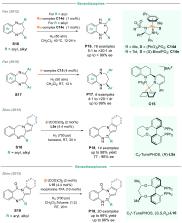
Chiral amines are key structural motifs present in a wide variety of natural products, drugs, and other biologically active compounds. During the past decade, significant advances have been made with respect to the enantioselective synthesis of chiral amines, many of them based on catalytic asymmetric hydrogenation (AH). The present review covers the use of AH in the synthesis of chiral amines bearing a stereogenic center either in the α, β, or γ position with respect to the nitrogen atom, reported from 2010 to 2020. Therefore, we provide an overview of the recent advances in the AH of imines, enamides, enamines, allyl amines, and N-heteroaromatic compounds.
Related collections
Most cited references556
- Record: found
- Abstract: found
- Article: not found
Fluorine in medicinal chemistry.
- Record: found
- Abstract: found
- Article: not found
Fluorine in pharmaceuticals: looking beyond intuition.
- Record: found
- Abstract: not found
- Article: not found