- Record: found
- Abstract: found
- Article: found
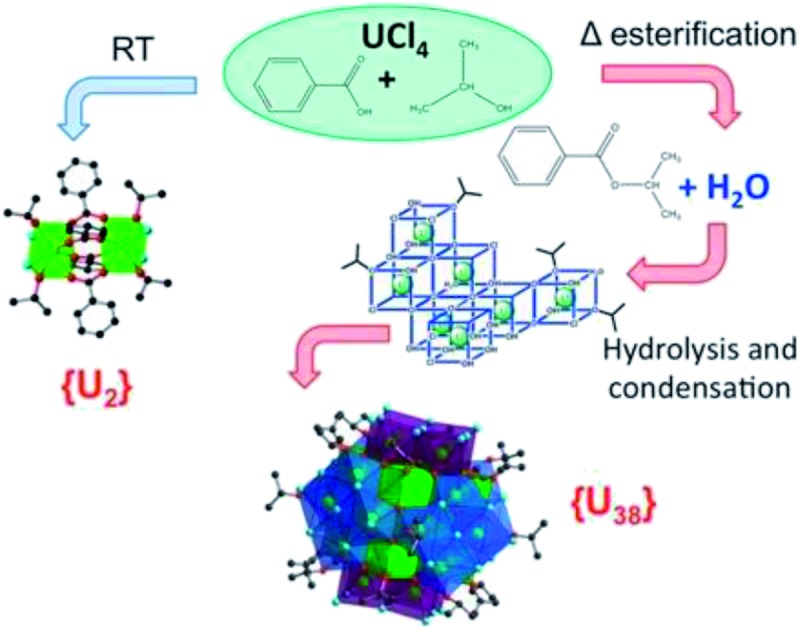
Formation of a new type of uranium(iv) poly-oxo cluster {U 38} based on a controlled release of water via esterification reaction†

Read this article at
Abstract
Abstract
A new strategy for the synthesis of large poly-oxo clusters bearing 38 tetravalent uranium atoms {U 38} has been developed by controlling the water release from the esterification reaction between a carboxylic acid and an alcohol. The molecular entity [U 38O 56Cl 40(H 2O) 2(ipa) 20]·(ipa) x (ipa = isopropanol) was crystallized from the solvothermal reaction of a mixture of UCl 4 and benzoic acid in isopropanol at temperature ranging from 70 to 130 °C. Its crystal structure reveals the molecular assembly of the UO 2 fluorite-like inner core {U 14} with oxo groups bridging the uranium centers. The {U 14} core is further surrounded by six tetrameric sub-units of {U 4} to form the {U 38} cluster. Its surface is decorated by either bridging- and terminal chloride anions or terminal isopropanol molecules. Another synthesis using the same reactant mixture at room temperature resulted in the crystallization of a discrete dinuclear complex [U 2Cl 4(bz) 4(ipa) 4]·(ipa) 0.5 (bz = benzoate), in which each uranium center is coordinated by two chlorine atoms, four oxygen atoms from carboxylate groups and two additional oxygen atoms from isopropanol. The slow production of water released from the esterification of isopropanol allows the formation of the giant cluster with oxo bridges linking the uranium atoms at a temperature above 70 °C, whereas no such oxo groups are present in the dinuclear complex formed at room temperature. The kinetics of {U 38} crystallization as well as the ester formation are analyzed and discussed. SAXS experiments indicate that the {U 38} species are not dominant in the supernatant, but hexanuclear entities which are closely related to the [U 6O 8] type are formed.
Related collections
Author and article information
Notes
†Electronic supplementary information (ESI) available: Optical photographs, SEM photographs and X-ray diffraction patterns of UO 2-like powders, UV/visible absorption spectra of supernatant solutions for 2, thermogravimetric curves, Avrami–Erofeev and Sharp-Hancock kinetic fits, UV-Vis spectra, and time evolution of 1H NMR spectra and SAXS curves (PDF). CCDC 1822435 and 1822436 crystallographic data for 1 and 2 (CIF). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc00752g