- Record: found
- Abstract: found
- Article: not found
Compact Polyelectrolyte Complexes: “Saloplastic” Candidates for Biomaterials

Read this article at
Abstract
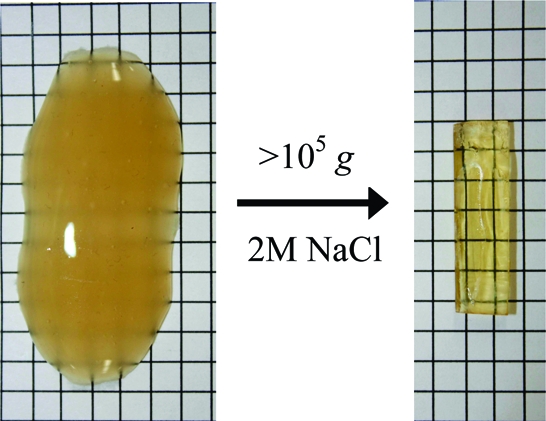
Precipitates of polyelectrolyte complexes were transformed into rugged shapes suitable for bioimplants by ultracentrifugation in the presence of high salt concentration. Salt ions dope the complex, creating a softer material with viscous fluid-like properties. Complexes that were compacted under the centrifugal field (CoPECs) were made from poly(diallyldimethyl ammonium), PDADMA, as polycation, and poly(styrene sulfonate), PSS, or poly(methacrylic acid), PMAA, as polyanion. Dynamic mechanical testing revealed a rubbery plateau at lower frequencies for PSS/PDADMA with moduli that decreased with increasing salt concentration, as internal ion pair cross-links were broken. CoPECs had significantly lower modulii compared to similar polyelectrolyte complexes prepared by the “multilayering” method. The difference in mechanical properties was ascribed to higher water content (located in micropores) for the former and, more importantly, to their nonstoichiometric polymer composition. The modulus of PMAA/PDADMA CoPECs, under physiological conditions, demonstrated dynamic mechanical properties that were close to those of the nucleus pulposus in an intervertebral disk.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Versatile Photocatalytic Systems for H2 Generation in Water Based on an Efficient DuBois-Type Nickel Catalyst
- Record: found
- Abstract: found
- Article: not found
The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules.
- Record: found
- Abstract: found
- Article: not found
