- Record: found
- Abstract: not found
- Article: not found
Update of the current role of ultrasound in asymptomatic hyperuricemia. A systematic literature review
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
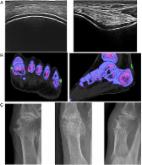
<p class="first" id="d4686757e141">Ultrasound (US) is a recognized imaging modality
for the assessment of gout. Recently
it is being explored for its potential role in the evaluation of subjects with asymptomatic
hyperuricemia (AH). Preliminary reports demonstrated the presence of monosodium urate
(MSU)-crystal deposits including aggregates, double contour sign and/or tophi in both
intra-articular and periarticular tissues of AH individuals. Although these results
are exciting, the value and potential application of US in AH remain to be clearly
delineated. In this systematic literature review, we aim to summarise the recent publications
regarding the role of US in the assessment of AH. We analyzed possible application
of US in the daily clinical practice and its future clinical and research potential
in the evaluation of AH individuals.
</p>
Related collections
Most cited references38

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
- Record: found
- Abstract: found
- Article: not found
QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies.
Penny F Whiting, Anne Rutjes, Marie E Westwood … (2011)
- Record: found
- Abstract: found
- Article: found
2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative
Tuhina Neogi, Tim Jansen, Nicola Dalbeth … (2015)