- Record: found
- Abstract: found
- Article: found
Vitamin D differentially regulates colon stem cells in patient‐derived normal and tumor organoids
Read this article at
Abstract
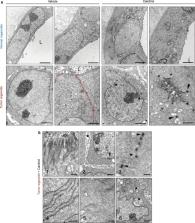
Intestine is a major target of vitamin D and several studies indicate an association between vitamin D deficiency and inflammatory bowel diseases ( IBD), but also increased colorectal cancer ( CRC) risk and mortality. However, the putative effects of 1α,25‐dihydroxyvitamin D 3 (calcitriol), the active vitamin D metabolite, on human colonic stem cells are unknown. Here we show by immunohistochemistry and RNAscope in situ hybridization that vitamin D receptor ( VDR) is unexpectedly expressed in LGR5 + colon stem cells in human tissue and in normal and tumor organoid cultures generated from patient biopsies. Interestingly, normal and tumor organoids respond differentially to calcitriol with profound and contrasting changes in their transcriptomic profiles. In normal organoids, calcitriol upregulates stemness‐related genes, such as LGR5 , SMOC2 , LRIG1 , MSI1 , PTK7 , and MEX3A , and inhibits cell proliferation. In contrast, in tumor organoids calcitriol has little effect on stemness‐related genes while it induces a differentiated phenotype, and variably reduces cell proliferation. Concordantly, electron microscopy showed that calcitriol does not affect the blastic undifferentiated cell phenotype in normal organoids but it induces a series of differentiated features in tumor organoids. Our results constitute the first demonstration of a regulatory role of vitamin D on human colon stem cells, indicating a homeostatic effect on colon epithelium with relevant implications in IBD and CRC.
Abstract
Vitamin D deficiency has been linked to enhanced colorectal cancer (CRC) risk and mortality. Human colonic stem cells, known as crypt stem cells, are essential in gut homeostasis, and their alteration is a feature of CRC. Here, Alberto Muñoz and colleagues generated a living‐biobank of stem‐cell‐derived normal and tumor colon organoids from CRC patients. Vitamin D upregulates stemness‐related genes and the undifferentiated phenotype in normal organoids, while it induces differentiation in tumor organoids. These findings uncover a regulatory role of vitamin D on crypt stem cells, with relevance to CRC development.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers.
- Record: found
- Abstract: found
- Article: not found
Isolation and in vitro expansion of human colonic stem cells.
- Record: found
- Abstract: found
- Article: not found