- Record: found
- Abstract: found
- Article: found
Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective
Read this article at
Abstract
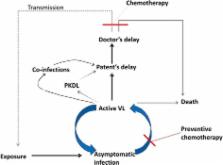
Visceral leishmaniasis (VL) is a serious parasitic disease causing considerable mortality and major disability in the Indian subcontinent. It is most neglected tropical disease, particularly in terms of new drug development for the lack of financial returns. An elimination campaign has been running in India since 2005 that aim to reduce the incidence of VL to below 1 per 10,000 people at sub-district level. One of the major components in this endeavor is reducing transmission through early case detection followed by complete treatment. Substantial progress has been made during the recent years in the area of VL treatment, and the VL elimination initiatives have already saved many lives by deploying them effectively in the endemic areas. However, many challenges remain to be overcome including availability of drugs, cost of treatment (drugs and hospitalization), efficacy, adverse effects, and growing parasite resistance. Therefore, better emphasis on implementation research is urgently needed to determine how best to deliver existing interventions with available anti-leishmanial drugs. It is essential that the new treatment options become truly accessible, not simply available in endemic areas so that they may promote healing and save lives. In this review, we highlight the recent advancement and challenges in current treatment options for VL in disease endemic area, and discuss the possible strategies to improve the therapeutic outcome.
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis.
- Record: found
- Abstract: found
- Article: not found
Oral miltefosine for Indian visceral leishmaniasis.
- Record: found
- Abstract: found
- Article: not found