- Record: found
- Abstract: found
- Article: found
Adverse reactions of targeted therapy in cancer patients: a retrospective study of hospital medical data in China
Read this article at
Abstract
Background
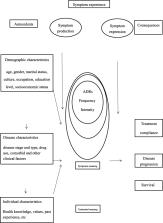
The adverse reactions (ADRs) of targeted therapy were closely associated with treatment response, clinical outcome, quality of life (QoL) of patients with cancer. However, few studies presented the correlation between ADRs of targeted therapy and treatment effects among cancer patients. This study was to explore the characteristics of ADRs with targeted therapy and the prognosis of cancer patients based on the clinical data.
Methods
A retrospective secondary data analysis was conducted within an ADR data set including 2703 patients with targeted therapy from three Henan medical centers of China between January 2018 and December 2019. The significance was evaluated with chi-square test between groups with or without ADRs. Univariate and multivariate logistic regression with backward stepwise method were applied to assess the difference of pathological characteristics in patients with cancer. Using the univariate Cox regression method, the actuarial probability of overall survival was performed to compare the clinical outcomes between these two groups.
Results
A total of 485 patients were enrolled in this study. Of all patients, 61.0% ( n = 296) occurred ADRs including skin damage, fatigue, mucosal damage, hypertension and gastrointestinal discomfort as the top 5 complications during the target therapy. And 62.1% of ADRs were mild to moderate, more than half of the ADRs occurred within one month, 68.6% ADRs lasted more than one month. Older patients ( P = 0.022) and patients with lower education level ( P = 0.036), more than 2 comorbidities ( P = 0.021), longer medication time ( P = 0.022), drug combination ( P = 0.033) and intravenous administration ( P = 0.019) were more likely to have ADRs. Those with ADRs were more likely to stop taking ( P = 0.000), change ( P = 0.000), adjust ( P = 0.000), or not take the medicine on time ( P = 0.000). The number of patients with recurrence ( P = 0.000) and metastasis ( P = 0.006) were statistically significant difference between ADRs and non-ADRs group. And the patients were significantly poor prognosis in ADRs groups compared with non-ADRs group.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries

- Record: found
- Abstract: found
- Article: found
Kinase-targeted cancer therapies: progress, challenges and future directions
- Record: found
- Abstract: found
- Article: not found