- Record: found
- Abstract: found
- Article: found
The SIDECAR project: S-IcD registry in European paediatriC and young Adult patients with congenital heaRt defects

Read this article at
Abstract
Aims
Subcutaneous-implantable cardiac defibrillators (S-ICDs) are used increasingly to prevent sudden cardiac death in young patients. This study was set up to gain insight in the indications for S-ICD, possible complications, and their predictors and follow-up results.
Methods and results
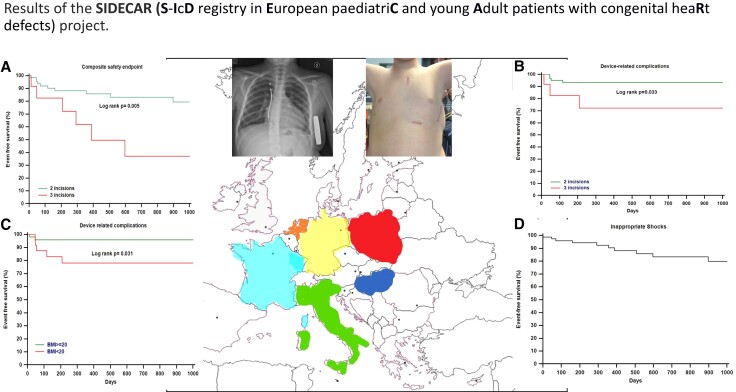
A multicentre, observational, retrospective, non-randomized, standard-of-care registry on S-ICD outcome in young patients with congenital heart diseases (CHDs), inherited arrhythmias (IAs), idiopathic ventricular fibrillation (IVF), and cardiomyopathies (CMPs). Anthropometry was registered as well as implantation technique, mid-term device-related complications, and incidence of appropriate/inappropriate shocks (IASs). Data are reported as median (interquartile range) or mean ± standard deviation. Eighty-one patients (47% CMPs, 20% CHD, 21% IVF, and 12% IA), aged 15 (14–17) years, with body mass index (BMI) 21.8 ± 3.8 kg/m 2, underwent S-ICD implantation (primary prevention in 59%). This was performed with two-incision technique in 81% and with a subcutaneous pocket in 59%. Shock and conditional zones were programmed at 250 (200–250) and 210 (180–240) b.p.m., respectively. No intraoperative complications occurred. Follow up was 19 (6–35) months: no defibrillation failure occurred, 17% of patients received appropriate shocks, 13% of patients received IAS (supraventricular tachycardias 40%, T-wave oversensing 40%, and non-cardiac oversensing 20%). Reprogramming, proper drug therapy, and surgical revision avoided further IAS. Complications requiring surgical revision occurred in 9% of patients, with higher risks in patients with three-incision procedures [hazard ratio (HR) 4.3, 95% confidence interval (95% CI) 0.5–34, P = 0.038] and BMI < 20 (HR 5.1, 95% CI 1–24, P = 0.031).
Graphical Abstract
Related collections
Most cited references20

- Record: found
- Abstract: found
- Article: found
Primary Results From the Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) Trial
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry.
- Record: found
- Abstract: found
- Article: not found