- Record: found
- Abstract: found
- Article: found
Combined Neuropeptide S and D-Cycloserine Augmentation Prevents the Return of Fear in Extinction-Impaired Rodents: Advantage of Dual versus Single Drug Approaches
Read this article at
Abstract
Background:
Despite its success in treating specific anxiety disorders, the effect of exposure therapy is limited by problems with tolerability, treatment resistance, and fear relapse after initial response. The identification of novel drug targets facilitating fear extinction in clinically relevant animal models may guide improved treatment strategies for these disorders in terms of efficacy, acceleration of fear extinction, and return of fear.
Methods:
The extinction-facilitating potential of neuropeptide S, D-cycloserine, and a benzodiazepine was investigated in extinction-impaired high anxiety HAB rats and 129S1/SvImJ mice using a classical cued fear conditioning paradigm followed by extinction training and several extinction test sessions to study fear relapse.
Results:
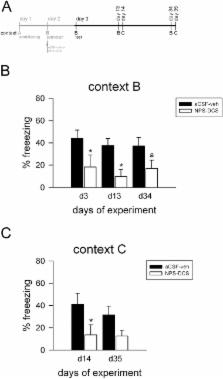
Administration of D-cycloserine improved fear extinction in extinction-limited, but not in extinction-deficient, rodents compared with controls. Preextinction neuropeptide S caused attenuated fear responses in extinction-deficient 129S1/SvImJ mice at extinction training onset and further reduced freezing during this session. While the positive effects of either D-cycloserine or neuropeptide S were not persistent in 129S1/SvImJ mice after 10 days, the combination of preextinction neuropeptide S with postextinction D-cycloserine rendered the extinction memory persistent and context independent up to 5 weeks after extinction training. This dual pharmacological adjunct to extinction learning also protected against fear reinstatement in 129S1/SvImJ mice.
Conclusions:
By using the potentially nonsedative anxiolytic neuropeptide S and the cognitive enhancer D-cycloserine to facilitate deficient fear extinction, we provide here the first evidence of a purported efficacy of a dual over a single drug approach. This approach may render exposure sessions less aversive and more efficacious for patients, leading to enhanced protection from fear relapse in the long term.
Related collections
Most cited references61
- Record: found
- Abstract: not found
- Article: not found
Emotional processing of fear: exposure to corrective information.
- Record: found
- Abstract: found
- Article: not found
Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity.
- Record: found
- Abstract: found
- Article: not found