- Record: found
- Abstract: found
- Article: found
Reduced TET2 function leads to T-cell lymphoma with follicular helper T-cell-like features in mice
Read this article at
Abstract
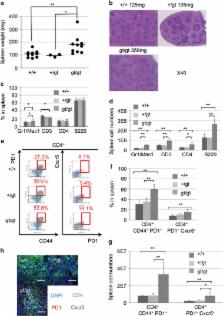
TET2 (Ten Eleven Translocation 2) is a dioxygenase that converts methylcytosine (mC) to hydroxymethylcytosine (hmC). TET2 loss-of-function mutations are highly frequent in subtypes of T-cell lymphoma that harbor follicular helper T (Tfh)-cell-like features, such as angioimmunoblastic T-cell lymphoma (30–83%) or peripheral T-cell lymphoma, not otherwise specified (10–49%), as well as myeloid malignancies. Here, we show that middle-aged Tet2 knockdown ( Tet2 gt/gt ) mice exhibit Tfh-like cell overproduction in the spleen compared with control mice. The Tet2 knockdown mice eventually develop T-cell lymphoma with Tfh-like features after a long latency (median 67 weeks). Transcriptome analysis revealed that these lymphoma cells had Tfh-like gene expression patterns when compared with splenic CD4-positive cells of wild-type mice. The lymphoma cells showed lower hmC densities around the transcription start site (TSS) and higher mC densities at the regions of the TSS, gene body and CpG islands. These epigenetic changes, seen in Tet2 insufficiency-triggered lymphoma, possibly contributed to predated outgrowth of Tfh-like cells and subsequent lymphomagenesis. The mouse model described here suggests that TET2 mutations play a major role in the development of T-cell lymphoma with Tfh-like features in humans.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study.
- Record: found
- Abstract: found
- Article: not found
Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found