- Record: found
- Abstract: found
- Article: found
Prostaglandin E 2 stimulates the epithelial sodium channel (ENaC) in cultured mouse cortical collecting duct cells in an autocrine manner
Read this article at
Abstract
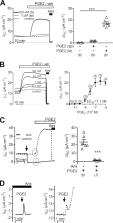
In murine cortical collecting duct cells, prostaglandin E 2 (PGE 2) stimulates transepithelial sodium transport mediated by the epithelial sodium channel (ENaC). PGE 2 is synthesized and secreted by the cells and acts on basolateral prostaglandin E receptor type 4 (EP 4).
Abstract
Prostaglandin E 2 (PGE 2) is the most abundant prostanoid in the kidney, affecting a wide range of renal functions. Conflicting data have been reported regarding the effects of PGE 2 on tubular water and ion transport. The amiloride-sensitive epithelial sodium channel (ENaC) is rate limiting for transepithelial sodium transport in the aldosterone-sensitive distal nephron. The aim of the present study was to explore a potential role of PGE 2 in regulating ENaC in cortical collecting duct (CCD) cells. Short-circuit current (I SC) measurements were performed using the murine mCCD cl1 cell line known to express characteristic properties of CCD principal cells and to be responsive to physiological concentrations of aldosterone and vasopressin. PGE 2 stimulated amiloride-sensitive I SC via basolateral prostaglandin E receptors type 4 (EP 4) with an EC 50 of ∼7.1 nM. The rapid stimulatory effect of PGE 2 on I SC resembled that of vasopressin. A maximum response was reached within minutes, coinciding with an increased abundance of β-ENaC at the apical plasma membrane and elevated cytosolic cAMP levels. The effects of PGE 2 and vasopressin were nonadditive, indicating similar signaling cascades. Exposing mCCD cl1 cells to aldosterone caused a much slower (∼2 h) increase of the amiloride-sensitive I SC. Interestingly, the rapid effect of PGE 2 was preserved even after aldosterone stimulation. Furthermore, application of arachidonic acid also increased the amiloride-sensitive I SC involving basolateral EP 4 receptors. Exposure to arachidonic acid resulted in elevated PGE 2 in the basolateral medium in a cyclooxygenase 1 (COX-1)–dependent manner. These data suggest that in the cortical collecting duct, locally produced and secreted PGE 2 can stimulate ENaC-mediated transepithelial sodium transport.
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
Prostanoid receptors: structures, properties, and functions.
- Record: found
- Abstract: found
- Article: not found
Epithelial sodium channels: function, structure, and regulation.
- Record: found
- Abstract: found
- Article: not found