- Record: found
- Abstract: found
- Article: found
Azide–Alkyne Cycloaddition Catalyzed by Copper(I) Coordination Polymers in PPM Levels Using Deep Eutectic Solvents as Reusable Reaction Media: A Waste-Minimized Sustainable Approach
Read this article at
Abstract

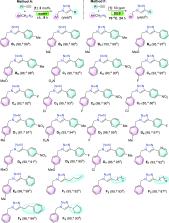
Two air-stable copper(I)–halide coordination polymers 1 and 2 with NNS and NNO ligand frameworks were synthesized and successfully utilized as efficient catalysts in an important organic reaction, namely, copper-catalyzed azide–alkyne cycloaddition, which is generally conducted in a mixture of water and organic solvents. The azide–alkyne “click” reaction was successfully conducted in pure water at r.t. under aerobic conditions. Other green solvents, including ethanol and glycerol, were also effectively used. Finally, deep eutectic solvents as green and sustainable reaction media were successfully utilized. In deep eutectic solvents, complete conversion with excellent isolated yield was achieved in a short period of time (1 h) with low catalyst loading (1 mol %) at r.t. Full conversion could also be achieved within 24 h with ppm-level (50 ppm) catalyst loading at 70 °C. Optimized reaction conditions were used for the syntheses of a large number of 1,4-disubstituted 1,2,3-triazoles with various functionalities. Triazole products were easily isolated by simple filtration. The reaction media, such as water and deep eutectic solvents, were recovered and recycled in three consecutive runs. The limited waste production is reflected in a very low E-factor (0.3–2.8). Finally, the CHEM21 green metrics toolkit was employed to evaluate the sustainability credentials of different optimized protocols in various green solvents such as water, ethanol, glycerol, and deep eutectic solvents.
Related collections
Most cited references104
- Record: found
- Abstract: not found
- Article: not found
Deep eutectic solvents (DESs) and their applications.
- Record: found
- Abstract: not found
- Article: not found
Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: spectroscopic data. See http://www.rsc.org/suppdata/cc/b2/b210714g/
- Record: found
- Abstract: found
- Article: not found