- Record: found
- Abstract: found
- Article: found
Production of Polyclonal Antibody against Grapevine fanleaf virus Movement Protein Expressed in Escherichia coli
Read this article at
Abstract
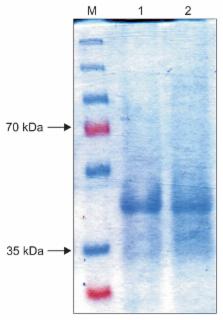
The genomic region of Grapevine fanleaf virus (GFLV) encoding the movement protein (MP) was cloned into pET21a and transformed into Escherichia coli strain BL21 (DE3) to express the protein. Induction was made with a wide range of isopropyl-β-D-thiogalactopyranoside (IPTG) concentrations (1, 1.5, and 2 mM) each for duration of 4, 6, or 16 h. However, the highest expression level was achieved with 1 mM IPTG for 4 h. Identity of the expressed protein was confirmed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. The expressed 41 kDa protein was purified under denaturing condition by affinity chromatography, reconfirmed by Western blotting and plate-trapped antigen enzyme-linked immunosorbent assay (PTA-ELISA) before being used as a recombinant antigen to raise polyclonal antibodies in rabbits. Purified anti-GFLV MP immunoglobulines (IgGs) and conjugated IgGs detected the expressed MP and GFLV virions in infected grapevines when used in PTA-ELISA, double antibody sandwich-ELISA, and Western blotting. This is the first report on the production of anti-GFLV MP polyclonal antibodies and application for the virus detection.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution.
- Record: found
- Abstract: found
- Article: not found
Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit.
- Record: found
- Abstract: found
- Article: not found