- Record: found
- Abstract: found
- Article: not found
The Role of HDAC6 in Cancer
Read this article at
Abstract
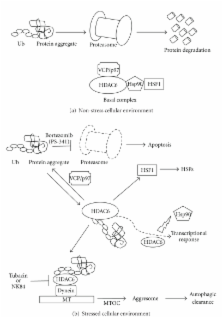
Histone deacetylase 6 (HDAC6), a member of the HDAC family whose major substrate is α-tubulin, has become a target for drug development to treat cancer due to its major contribution in oncogenic cell transformation. Overexpression of HDAC6 correlates with tumorigenesis and improved survival; therefore, HDAC6 may be used as a marker for prognosis. Previous work demonstrated that in multiple myeloma cells, inhibition of HDAC6 results in apoptosis. Furthermore, HDAC6 is required for the activation of heat-shock factor 1 (HSF1), an activator of heat-shock protein encoding genes (HSPs) and CYLD, a cylindromatosis tumor suppressor gene. HDAC6 contributes to cancer metastasis since its upregulation increases cell motility in breast cancer MCF-7 cells and its interaction with cortactin regulates motility. HDAC6 also affects transcription and translation by regulating the heat-shock protein 90 (Hsp90) and stress granules (SGs), respectively. This review will discuss the role of HDAC6 in the pathogenesis and treatment of cancer.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Histone deacetylases and cancer: causes and therapies.
- Record: found
- Abstract: found
- Article: not found
Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors.
- Record: found
- Abstract: found
- Article: not found
