- Record: found
- Abstract: found
- Article: found
Pharmacokinetics of Dexamethasone when Administered with Fosaprepitant for Chemotherapy-Induced Nausea and Vomiting and Differences in Dose-Dependent Antiemetic Effects
Read this article at
Abstract
Background:
Fosaprepitant, an NK 1 receptor antagonist, inhibits and induces cytochrome P450 3A4 (CYP3A4) as its substrate. Contrarily dexamethasone is metabolized by CYP3A4. Therefore, in combination therapy wherein both agents interact with each other, it is recommended that the dexamethasone dose be reduced in the first two days. Thus far, there are only a few studies on the optimum dose of dexamethasone after day 3. Thus, we aimed to determine the pharmacokinetics of dexamethasone on day3 when administered together with fosaprepitant and investigate the dose-dependent differences in its antiemetic effect in patients with cancer.
Methods:
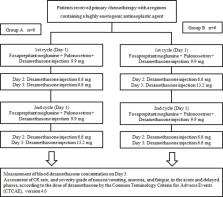
Twelve patients with esophageal, stomach, or lung cancer received primary highly emetogenic chemotherapy (HEC). We intravenously administered 9.9 mg and 6.6 mg of dexamethasone on days 1 and 2, respectively, and 6.6 mg or 13.2 mg on day 3 together with the administration of 150 mg fosaprepitant and 0.75 mg palonosetron. We assessed the pharmacokinetics of dexamethasone on day 3 by dose and examined the dose-dependent antiemetic effect.
Results:
No differences were observed in the time-to-maximum concentration and blood half-life of dexamethasone between patient groups that received dexamethasone at doses of 6.6 mg and 13.2 mg. In contrast, the area under the blood concentration-time curve and the maximum concentration of dexamethasone correlated with its dose. Moreover, the blood dexamethasone concentration on day 3 increased by twofold after the administration of a higher dose than after a lower dose. The severity of nausea in the delayed phase significantly decreased in a dose-dependent manner.
Related collections
Most cited references9
- Record: found
- Abstract: not found
- Article: not found
2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients.
- Record: found
- Abstract: found
- Article: not found
Antiemetics: ASCO Guideline Update
- Record: found
- Abstract: found
- Article: not found