- Record: found
- Abstract: found
- Article: found
Regulation of extrathymic Treg cell conversion by CD5
editorial
26 September 2015
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The immune system must carefully regulate the balance between immunity and tolerance
in order to prevent disease. Mechanisms of tolerance include crucial functions of
thymically developed regulatory T (tTreg) cells as well as peripheral regulatory T
(pTreg) cells that differentiate from T cells outside the thymus [1]. The development
of pTreg cells is tightly regulated to promote tolerance to innocuous and self-antigens
without compromising the ability of the immune system to remove offending pathogens.
The autoimmune diseases can be caused by decreased functions of Treg cells but anti-tumor
responses can instead be hampered by aberrant immune regulation. Many promising therapies
revolve around correcting such imbalances of Treg cell functions. Therefore, the current
intense research efforts to elucidate the mechanisms governing Treg cell differentiation
could lead to new therapies to alleviate autoimmunity, inflammatory diseases and cancer
[1, 2].
During thymic T cell development, T cells that respond strongly to self-antigens increase
their CD5 expression to parallel the T cell receptor (TCR) signal strength and such
T cells become CD5hi [3]. Further, previous findings also revealed that some T cells
that initially remained CD5lo during thymic selection can still up regulate their
CD5 expression outside the thymus in response to cognate self-antigens presented by
peripheral tolerogenic dendritic cells (DCs) [4]. Consistent with CD5 expression correlating
with TCR signal strength in thymus, expression of CD5 is also increased in regulatory
T cells although CD5 is not required for the development of tTreg cells [5]. While
the majority of CD5hi cells in thymus do not develop into tTreg cells, the elevated
CD5 expression persists in mature peripheral T cells to distinguish CD5hi and CD5lo
T cells that responded with, respectively, high or low affinity to self-peptide(p)MHCs
in thymus [6]. Despite the functions of CD5 as a negative regulator of TCR signaling,
the CD5hi T cells remain responsive to antigenic stimulation and are capable of forming
effector T cells that are cross-reactive to self antigens thereby risking the development
of anti-self responses [6]. The question then arises: how are such self-reactive T
cells specifically instructed to convert into pTreg cells to help provide an antigen-specific
tolerance?
Recently, we discovered a CD5-dependent mechanism to promote the conversion of self-reactive
peripheral CD5hi T cells into extrathymic Treg cells that block autoimmunity [7] and
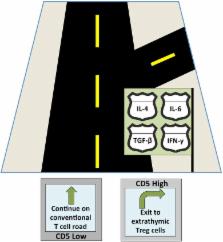
Figure 1. We found that CD5 promotes the conversion of such CD5hi cells into Foxp3+
pTreg cells by blocking the mechanistic target of rapamycin (mTOR) activated in response
to effector T cell-differentiating cytokines such as IL-4, IL-6, and IFN-γ [7]. These
findings indicate that CD5hi cells are less susceptible to the effector cytokine-mediated
inhibition of Treg cell differentiation and therefore self-reactive CD5hi T cells
might preferentially convert into pTreg cells even during an on-going inflammatory
process to alleviate autoimmunity. In contrast, CD5lo T cells, which are not reactive
to self or innocuous peripheral antigens but may be specific for foreign pathogens,
have decreased conversion into Treg cells in the absence of CD5 functions [7]. Overall,
CD5 regulates a selective extrathymic induction of Treg cells from T cells that have
responded to high affinity self-pMHC in thymus or tolerizing antigens presented by
tolerogenic DCs in the periphery, without compromising the general high plasticity
of immune responses among the total T cell repertoire.
Figure 1
CD5 serves as a T cell “guidance system” navigating T cells towards Treg cell differentiation
despite the presence of various cytokines that each signal a different developmental
fate (represented by the confusing road signs).
The role of CD5 in promoting tolerance to self-reactive antigens could also be exploited
for future immune therapies. A selective increase of CD5 functions in autoaggressive
T cells could enable careful targeting of tolerance thus avoiding general immunosuppression
that is often associated with current immunomodulatory therapies. Additionally, tumor
microenvironments are characterized by increased numbers of Treg cells that may prevent
rejections of tumors despite the on-going pro-inflammatory process [2]. Since CD5
functions can enable the differentiation of pTreg cells despite the presence of effector
T cell cytokines, it is attractive to speculate that tumors might manipulate the CD5
expression in responding T cells in a mechanism to skew T cell differentiation into
pTreg cells, which then contribute to tumor survival. Disrupting such upregulation
of CD5 in tumor-specific T cells could therefore prevent tumor cells from generating
Treg cells necessary to block rejection of the tumor by the immune system without
increasing the risk of autoaggressive immune responses. Undoubtedly, in order to develop
such treatments, more work must be done to understand the mechanisms regulating expression
and functions of CD5 in T cells. Doing so will also provide an insight into which
pathways could become most promising targets for future immune therapies.
Related collections
Most cited references3
- Record: found
- Abstract: found
- Article: not found
Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo.
- Record: found
- Abstract: found
- Article: not found
CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens.
Jacob Henderson, Adeleye Opejin, Andrew Jones … (2015)
- Record: found
- Abstract: found
- Article: not found
CD5 plays an inhibitory role in the suppressive function of murine CD4(+) CD25(+) T(reg) cells.
Joseph Qualls, H Tuna, Trivikram Dasu … (2008)