- Record: found
- Abstract: found
- Article: found
Brain Dopamine Transmission in Health and Parkinson's Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors
Read this article at
Abstract
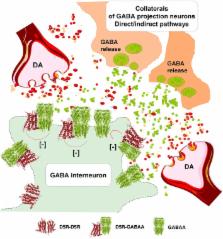
This perspective article provides observations supporting the view that nigro-striatal dopamine neurons and meso-limbic dopamine neurons mainly communicate through short distance volume transmission in the um range with dopamine diffusing into extrasynaptic and synaptic regions of glutamate and GABA synapses. Based on this communication it is discussed how volume transmission modulates synaptic glutamate transmission onto the D1R modulated direct and D2R modulated indirect GABA pathways of the dorsal striatum. Each nigro-striatal dopamine neuron was first calculated to form large numbers of neostriatal DA nerve terminals and then found to give rise to dense axonal arborizations spread over the neostriatum, from which dopamine is released. These neurons can through DA volume transmission directly influence not only the striatal GABA projection neurons but all the striatal cell types in parallel. It includes the GABA nerve cells forming the island-/striosome GABA pathway to the nigral dopamine cells, the striatal cholinergic interneurons and the striatal GABA interneurons. The dopamine modulation of the different striatal nerve cell types involves the five dopamine receptor subtypes, D1R to D5R receptors, and their formation of multiple extrasynaptic and synaptic dopamine homo and heteroreceptor complexes. These features of the nigro-striatal dopamine neuron to modulate in parallel the activity of practically all the striatal nerve cell types in the dorsal striatum, through the dopamine receptor complexes allows us to understand its unique and crucial fine-tuning of movements, which is lost in Parkinson's disease. Integration of striatal dopamine signals with other transmitter systems in the striatum mainly takes place via the receptor-receptor interactions in dopamine heteroreceptor complexes. Such molecular events also participate in the integration of volume transmission and synaptic transmission. Dopamine modulation of the glutamate synapses on the dorsal striato-pallidal GABA pathway involves D2R heteroreceptor complexes such as D2R-NMDAR, A2AR-D2R, and NTSR1-D2R heteroreceptor complexes. The dopamine modulation of glutamate synapses on the striato-entopeduncular/nigral pathway takes place mainly via D1R heteroreceptor complexes such as D1R-NMDAR, A2R-D1R, and D1R-D3R heteroreceptor complexes. Dopamine modulation of the island/striosome compartment of the dorsal striatum projecting to the nigral dopamine cells involve D4R-MOR heteroreceptor complexes. All these receptor-receptor interactions have relevance for Parkinson's disease and its treatment.
Related collections
Most cited references221
- Record: found
- Abstract: found
- Article: not found
The glutamate homeostasis hypothesis of addiction.
- Record: found
- Abstract: found
- Article: not found
Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory.
- Record: found
- Abstract: found
- Article: not found