- Record: found
- Abstract: found
- Article: found
Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids
Read this article at
Abstract
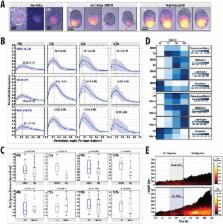
The establishment of the anteroposterior (AP) axis is a crucial step during animal embryo development. In mammals, genetic studies have shown that this process relies on signals spatiotemporally deployed in the extra-embryonic tissues that locate the position of the head and the onset of gastrulation, marked by T/Brachyury ( T/Bra) at the posterior of the embryo. Here, we use gastruloids, mESC-based organoids, as a model system with which to study this process. We find that gastruloids localise T/Bra expression to one end and undergo elongation similar to the posterior region of the embryo, suggesting that they develop an AP axis. This process relies on precisely timed interactions between Wnt/β-catenin and Nodal signalling, whereas BMP signalling is dispensable. Additionally, polarised T/Bra expression occurs in the absence of extra-embryonic tissues or localised sources of signals. We suggest that the role of extra-embryonic tissues in the mammalian embryo might not be to induce the axes but to bias an intrinsic ability of the embryo to initially break symmetry. Furthermore, we suggest that Wnt signalling has a separable activity involved in the elongation of the axis.
Abstract
[Related article:] Highlighted Article: Early gastruloid patterning involves the development of the embryonic axes in the absence of extra-embryonic tissues, a process that relies on the interactions of Nodal and Wnt, but not BMP.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling.
- Record: found
- Abstract: found
- Article: not found
Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture.
- Record: found
- Abstract: found
- Article: not found