- Record: found
- Abstract: found
- Article: found
Read this article at
Abstract
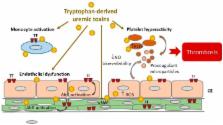
Patients with chronic kidney disease (CKD) display an elevated risk of thrombosis. Thrombosis occurs in cardiovascular events, such as venous thromboembolism, stroke, and acute coronary syndrome, and is a cause of hemodialysis vascular access dysfunction. CKD leads to the accumulation of uremic toxins, which exerts toxic effects on blood and the vessel wall. Some uremic toxins result from tryptophan metabolization in the gut through the indolic and the kynurenine pathways. An increasing number of studies are highlighting the link between such uremic toxins and thrombosis in CKD. In this review, we describe the thrombotic mechanisms induced by tryptophan-derived uremic toxins (TDUT). These mechanisms include an increase in plasma levels of procoagulant factors, induction of platelet hyperactivity, induction of endothelial dysfunction/ impairment of endothelial healing, decrease in nitric oxide (NO) bioavailability, and production of procoagulant microparticles. We focus on one important prothrombotic mechanism: The induction of tissue factor (TF), the initiator of the extrinsic pathway of the blood coagulation. This induction occurs via a new pathway, dependent on the transcription factor Aryl hydrocarbon receptor (AhR), the receptor of TDUT in cells. A better understanding of the prothrombotic mechanisms of uremic toxins could help to find novel therapeutic targets to prevent thrombosis in CKD.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline.
- Record: found
- Abstract: found
- Article: not found
Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality.
- Record: found
- Abstract: found
- Article: not found