- Record: found
- Abstract: found
- Article: found
A new design for a green calcium indicator with a smaller size and a reduced number of calcium-binding sites
Read this article at
Abstract
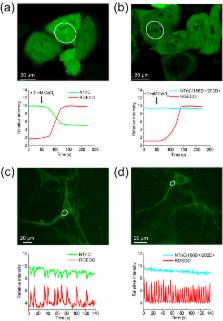
Genetically encoded calcium indicators (GECIs) are mainly represented by two- or one-fluorophore-based sensors. One type of two-fluorophore-based sensor, carrying Opsanus troponin C (TnC) as the Ca 2+-binding moiety, has two binding sites for calcium ions, providing a linear response to calcium ions. One-fluorophore-based sensors have four Ca 2+-binding sites but are better suited for in vivo experiments. Herein, we describe a novel design for a one-fluorophore-based GECI with two Ca 2+-binding sites. The engineered sensor, called NTnC, uses TnC as the Ca 2+-binding moiety, inserted in the mNeonGreen fluorescent protein. Monomeric NTnC has higher brightness and pH-stability in vitro compared with the standard GECI GCaMP6s. In addition, NTnC shows an inverted fluorescence response to Ca 2+. Using NTnC, we have visualized Ca 2+ dynamics during spontaneous activity of neuronal cultures as confirmed by control NTnC and its mutant, in which the affinity to Ca 2+ is eliminated. Using whole-cell patch clamp, we have demonstrated that NTnC dynamics in neurons are similar to those of GCaMP6s and allow robust detection of single action potentials. Finally, we have used NTnC to visualize Ca 2+ neuronal activity in vivo in the V1 cortical area in awake and freely moving mice using two-photon microscopy or an nVista miniaturized microscope.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin.
- Record: found
- Abstract: found
- Article: not found
A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum
- Record: found
- Abstract: found
- Article: not found