- Record: found
- Abstract: found
- Article: found
Variations in local calcium signaling in adjacent cardiac myocytes of the intact mouse heart detected with two-dimensional confocal microscopy
Read this article at
Abstract
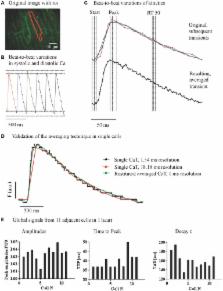
Dyssynchronous local Ca release within individual cardiac myocytes has been linked to cellular contractile dysfunction. Differences in Ca kinetics in adjacent cells may also provide a substrate for inefficient contraction and arrhythmias. In a new approach we quantify variation in local Ca transients between adjacent myocytes in the whole heart. Langendorff-perfused mouse hearts were loaded with Fluo-8 AM to detect Ca and Di-4-ANEPPS to visualize cell membranes. A spinning disc confocal microscope with a fast camera allowed us to record Ca signals within an area of 465 μm by 315 μm with an acquisition speed of 55 fps. Images from multiple transients recorded at steady state were registered to their time point in the cardiac cycle to restore averaged local Ca transients with a higher temporal resolution. Local Ca transients within and between adjacent myocytes were compared with regard to amplitude, time to peak and decay at steady state stimulation (250 ms cycle length). Image registration from multiple sequential Ca transients allowed reconstruction of high temporal resolution (2.4 ± 1.3 ms) local CaT in 2D image sets ( N = 4 hearts, n = 8 regions). During steady state stimulation, spatial Ca gradients were homogeneous within cells in both directions and independent of distance between measured points. Variation in CaT amplitudes was similar across the short and the long side of neighboring cells. Variations in TAU and TTP were similar in both directions. Isoproterenol enhanced the CaT but not the overall pattern of spatial heterogeneities. Here we detected and analyzed local Ca signals in intact mouse hearts with high temporal and spatial resolution, taking into account 2D arrangement of the cells. We observed significant differences in the variation of CaT amplitude along the long and short axis of cardiac myocytes. Variations of Ca signals between neighboring cells may contribute to the substrate of cardiac remodeling.
Related collections
Most cited references34

- Record: found
- Abstract: found
- Article: found
Remodelling of gap junctions and connexin expression in diseased myocardium
- Record: found
- Abstract: found
- Article: not found
So little source, so much sink: requirements for afterdepolarizations to propagate in tissue.
- Record: found
- Abstract: found
- Article: not found