- Record: found
- Abstract: found
- Article: found
The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance - a randomized, double-blind, placebo-controlled, crossover clinical trial
Read this article at
Abstract
Background
Lactose intolerance is a form of lactose maldigestion where individuals experience symptoms such as diarrhea, abdominal cramping, flatulence, vomiting and bowel sounds following lactose consumption. Lactobacillus acidophilus is a species of bacteria known for its sugar fermenting properties. Preclinical studies have found that Lactobacillus acidophilus supplementation may assist in breaking down lactose; however, no human clinical trials exist evaluating its efficacy in alleviating symptoms related to lactose intolerance.
Objective
The aim of this randomized, double-blind, placebo-controlled, crossover study was to evaluate the effect of a proprietary strain of Lactobacillus acidophilus on relieving discomfort related to lactose intolerance.
Methods
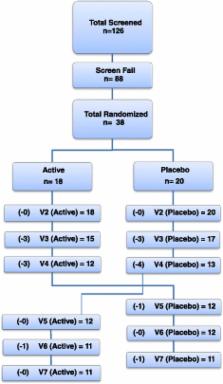
The study enrolled healthy volunteers between 18 and 75 years of age who complained of lactose intolerance. Screening visits included a lactose challenge visit to confirm eligibility based on a score of 10 or higher on subjective assessment of the following symptoms after lactose challenge: diarrhea, abdominal cramping, vomiting, audible bowel sounds, flatulence, and overall symptoms. Qualified subjects participated in a 2-arm crossover design, with each arm consisting of 4 weeks of intervention of either active or placebo product, with a 2-week washout period during crossover. The study product consisted of the DDS-1 strain of Lactobacillus acidophilus (Nebraska Cultures, Walnut Creek, California). The placebo was formulated from maltodextrin. Study participants were instructed to take the product once daily for 4 weeks. Data collected included subjective symptom scores related to lactose intolerance.
Results
Longitudinal comparison between the DDS-1 group and placebo group demonstrated statistically significant reductions in abdominal symptom scores during the 6-h Lactose Challenge at week 4 for diarrhea ( p = 0.033), abdominal cramping ( p = 0.012), vomiting ( p = 0.0002), and overall symptom score ( p = 0.037). No adverse events were reported.
Related collections
Most cited references50
- Record: found
- Abstract: not found
- Article: not found
Lactose Intolerance
- Record: found
- Abstract: found
- Article: not found
Systematic review: effective management strategies for lactose intolerance.
- Record: found
- Abstract: found
- Article: not found