- Record: found
- Abstract: found
- Article: found
NAD metabolic therapy in metabolic dysfunction-associated steatotic liver disease: Possible roles of gut microbiota
Read this article at
Summary
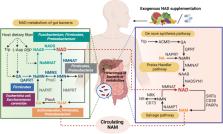
Metabolic dysfunction–associated steatotic liver disease (MASLD), formerly named non-alcoholic fatty liver disease (NAFLD), is induced by alterations of hepatic metabolism. As a critical metabolites function regulator, nicotinamide adenine dinucleotide (NAD) nowadays has been validated to be effective in the treatment of diet-induced murine model of MASLD. Additionally, gut microbiota has been reported to have the potential to prevent MASLD by dietary NAD precursors metabolizing together with mammals. However, the underlying mechanism remains unclear. In this review, we hypothesized that NAD enhancing mitochondrial activity might reshape a specific microbiota signature, and improve MASLD progression demonstrated by fecal microbiota transplantation. Here, this review especially focused on the mechanism of Microbiota-Gut-Liver Axis together with NAD metabolism for the MASLD progress. Notably, we found significant changes in Prevotella associated with NAD in a gut microbiome signature of certain MASLD patients. With the recent researches, we also inferred that Prevotella can not only regulate the level of NAD pool by boosting the carbon metabolism, but also play a vital part in regulating the branched-chain amino acid (BCAA)-related fatty acid metabolism pathway. Altogether, our results support the notion that the gut microbiota contribute to the dietary NAD precursors metabolism in MASLD development and the dietary NAD precursors together with certain gut microbiota may be a preventive or therapeutic strategy in MASLD management.
Graphical abstract
Abstract
Microbiome; Microbial metabolism
Related collections
Most cited references126
- Record: found
- Abstract: found
- Article: not found
Mechanisms of NAFLD development and therapeutic strategies
- Record: found
- Abstract: found
- Article: not found
NAFLD: a multisystem disease.
- Record: found
- Abstract: found
- Article: not found
