- Record: found
- Abstract: found
- Article: found
Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase
Read this article at
Abstract
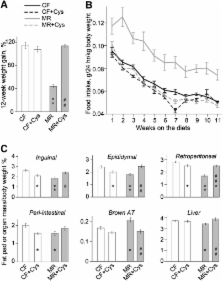
Stearoyl-CoA desaturase-1 (SCD1) is a key enzyme in fatty acid and energy metabolism, but little is known about its nutritional regulation. Dietary methionine restriction in rats decreases hepatic Scd1 mRNA and protein, increases energy expenditure, and decreases fat-pad mass/body-weight% (FM/BW%). In humans, plasma concentrations of the methionine product, cysteine, are associated with obesity. To determine which consequences of methionine-restriction are mediated by decreased cysteine availability, we monitored obesity-related variables in 4 dietary groups for 12 weeks: control-fed (CF), methionine-restricted (MR), MR supplemented with 0.5% l-cysteine (MR+Cys) and CF+Cys rats. MR lowered weight gain and FM/BW% despite higher food intake/weight than CF, and lowered serum cysteine. Hepatic Scd1 expression was decreased, with decreased serum SCD1 activity indices (calculated from serum fatty acid profile), decreased serum insulin, leptin and triglycerides, and higher adiponectin. Cysteine supplementation (MR+Cys) essentially reversed all these phenotypes and raised serum cysteine but not methionine to CF levels. Adding extra cysteine to control diet (CF+Cys) increased serum taurine but did not affect serum cysteine, lipids, proteins, or total weight gain. FM/BW% and serum leptin were modestly decreased. Our results indicate that anti-obesity effects of MR are caused by low cysteine and that dietary sulfur amino acid composition contributes to SCD1 regulation.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review.
- Record: found
- Abstract: found
- Article: not found
Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism.
- Record: found
- Abstract: found
- Article: found