- Record: found
- Abstract: found
- Article: found
The state of the art of bispecific antibodies for treating human malignancies
review-article
Shuhang Wang
1 ,
Kun Chen
2 ,
Qi Lei
1 ,
Peiwen Ma
1 ,
Andy Qingan Yuan
3
,
4 ,
Yong Zhao
5 ,
Youwei Jiang
6 ,
Hong Fang
1 ,
Shujun Xing
1 ,
Yuan Fang
1 ,
Ning Jiang
1 ,
Huilei Miao
1 ,
Minghui Zhang
7 ,
Shujun Sun
8 ,
Zicheng Yu
9 ,
Wei Tao
10 ,
Qi Zhu
10 ,
Yingjie Nie
2
,
,
Ning Li
1
,
24 August 2021
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
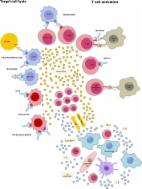
Bispecific antibodies (bsAb) that target two independent epitopes or antigens have been extensively explored in translational and clinical studies since they were first developed in the 1960s. Many bsAbs are being tested in clinical trials for treating a variety of diseases, mostly cancer. Here, we provide an overview of various types of bsAbs in clinical studies and discuss their targets, safety profiles, and efficacy. We also highlight the current challenges, potential solutions, and future directions of bsAb development for cancer treatment.
Abstract
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of T cell co-stimulation and co-inhibition.
Lieping Chen, Dallas Flies (2013)
- Record: found
- Abstract: found
- Article: not found
4-1BB Costimulation Ameliorates T Cell Exhaustion Induced by Tonic Signaling of Chimeric Antigen Receptors
Adrienne H Long, Waleed M. Haso, Jack F. Shern … (2015)