- Record: found
- Abstract: found
- Article: found
Four Ca 2+ Ions Activate TRPM2 Channels by Binding in Deep Crevices near the Pore but Intracellularly of the Gate
Read this article at
Abstract
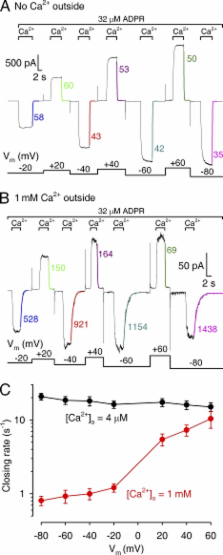
TRPM2 is a tetrameric Ca 2+-permeable channel involved in immunocyte respiratory burst and in postischaemic neuronal death. In whole cells, TRPM2 activity requires intracellular ADP ribose (ADPR) and intra- or extracellular Ca 2+, but the mechanism and the binding sites for Ca 2+ activation remain unknown. Here we study TRPM2 gating in inside-out patches while directly controlling intracellular ligand concentrations. Concentration jump experiments at various voltages and Ca 2+ dependence of steady-state single-channel gating kinetics provide unprecedented insight into the molecular mechanism of Ca 2+ activation. In patches excised from Xenopus laevis oocytes expressing human TRPM2, coapplication of intracellular ADPR and Ca 2+ activated ∼50-pS nonselective cation channels; K 1/2 for ADPR was ∼1 µM at saturating Ca 2+. Intracellular Ca 2+ dependence of TRPM2 steady-state opening and closing rates (at saturating [ADPR] and low extracellular Ca 2+) reveals that Ca 2+ activation is a consequence of tighter binding of Ca 2+ in the open rather than in the closed channel conformation. Four Ca 2+ ions activate TRPM2 with a Monod-Wymann-Changeux mechanism: each binding event increases the open-closed equilibrium constant ∼33-fold, producing altogether 10 6-fold activation. Experiments in the presence of 1 mM of free Ca 2+ on the extracellular side clearly show that closed channels do not sense extracellular Ca 2+, but once channels have opened Ca 2+ entering passively through the pore slows channel closure by keeping the “activating sites” saturated, despite rapid continuous Ca 2+-free wash of the intracellular channel surface. This effect of extracellular Ca 2+ on gating is gradually lost at progressively depolarized membrane potentials, where the driving force for Ca 2+ influx is diminished. Thus, the activating sites lie intracellularly from the gate, but in a shielded crevice near the pore entrance. Our results suggest that in intact cells that contain micromolar ADPR a single brief puff of Ca 2+ likely triggers prolonged, self-sustained TRPM2 activity.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches.
- Record: found
- Abstract: found
- Article: not found
Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs.
- Record: found
- Abstract: found
- Article: not found